Comparative neuropathogenesis and neurovirulence of attenuated flaviviruses in nonhuman primates
- PMID: 18353947
- PMCID: PMC2395187
- DOI: 10.1128/JVI.00172-08
Comparative neuropathogenesis and neurovirulence of attenuated flaviviruses in nonhuman primates
Abstract
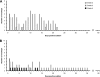
Based on previous preclinical evaluation in mice and monkeys, the chimeric TBEV/DEN4Delta30 virus, carrying the prM and E protein genes from a highly virulent Far Eastern strain of tick-borne encephalitis virus (TBEV) on the backbone of a nonneuroinvasive dengue type 4 virus (DEN4), has been identified as a promising live attenuated virus vaccine candidate against disease caused by TBEV. However, prior to use of this vaccine candidate in humans, its neurovirulence in nonhuman primates needed to be evaluated. In the present study, we compared the neuropathogeneses of the chimeric TBEV/DEN4Delta30 virus; Langat virus (LGTV), a former live TBEV vaccine; and yellow fever 17D virus vaccine (YF 17D) in rhesus monkeys inoculated intracerebrally. TBEV/DEN4Delta30 and YF 17D demonstrated remarkably similar spatiotemporal profiles of virus replication and virus-associated histopathology in the central nervous system (CNS) that were high in cerebral hemispheres but progressively decreased toward the spinal cord. In contrast, the neurovirulence of LGTV exhibited the reverse profile, progressing from the site of inoculation toward the cerebellum and spinal cord. Analysis of the spatiotemporal distribution of viral antigens in the CNS of monkeys revealed a prominent neurotropism associated with all three attenuated viruses. Nevertheless, TBEV/DEN4Delta30 virus exhibited higher neurovirulence in monkeys than either LGTV or YF 17D, suggesting insufficient attenuation. These results provide insight into the neuropathogenesis associated with attenuated flaviviruses that may guide the design of safe vaccines.
Figures




Comment in
-
Neuropathogenesis and neurovirulence of live flaviviral vaccines in monkeys.J Virol. 2009 May;83(10):5289-90; author reply 5290-2. doi: 10.1128/JVI.02621-08. J Virol. 2009. PMID: 19383635 Free PMC article. No abstract available.
Similar articles
-
MicroRNA-based control of tick-borne flavivirus neuropathogenesis: Challenges and perspectives.Antiviral Res. 2016 Mar;127:57-67. doi: 10.1016/j.antiviral.2016.01.003. Epub 2016 Jan 19. Antiviral Res. 2016. PMID: 26794396 Free PMC article.
-
Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys.Vaccine. 2006 Jan 12;24(2):133-43. doi: 10.1016/j.vaccine.2005.07.067. Epub 2005 Aug 9. Vaccine. 2006. PMID: 16115704
-
Stable and Highly Immunogenic MicroRNA-Targeted Single-Dose Live Attenuated Vaccine Candidate against Tick-Borne Encephalitis Constructed Using Genetic Backbone of Langat Virus.mBio. 2019 Apr 23;10(2):e02904-18. doi: 10.1128/mBio.02904-18. mBio. 2019. PMID: 31015334 Free PMC article.
-
Chimeric flaviviruses: novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis.Adv Virus Res. 2003;61:469-509. doi: 10.1016/s0065-3527(03)61013-4. Adv Virus Res. 2003. PMID: 14714441 Review.
-
Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis.Virus Res. 2005 Aug;111(2):161-74. doi: 10.1016/j.virusres.2005.04.007. Virus Res. 2005. PMID: 15871909 Review.
Cited by
-
Effect of a single point mutation on equine herpes virus 9 (EHV-9) neuropathogenicity after intranasal inoculation in a hamster model.J Vet Med Sci. 2017 Aug 18;79(8):1426-1436. doi: 10.1292/jvms.17-0076. Epub 2017 Jul 15. J Vet Med Sci. 2017. PMID: 28717112 Free PMC article.
-
Spatiotemporal profile of an optimal host response to virus infection in the primate central nervous system.PLoS Pathog. 2025 Jan 22;21(1):e1012530. doi: 10.1371/journal.ppat.1012530. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39841753 Free PMC article.
-
Virus infection of the CNS disrupts the immune-neural-synaptic axis via induction of pleiotropic gene regulation of host responses.Elife. 2021 Feb 18;10:e62273. doi: 10.7554/eLife.62273. Elife. 2021. PMID: 33599611 Free PMC article.
-
Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise.Vaccines (Basel). 2020 Aug 12;8(3):451. doi: 10.3390/vaccines8030451. Vaccines (Basel). 2020. PMID: 32806696 Free PMC article. Review.
-
Chimeric tick-borne encephalitis/dengue virus is attenuated in Ixodes scapularis ticks and Aedes aegypti mosquitoes.Vector Borne Zoonotic Dis. 2011 Jun;11(6):665-74. doi: 10.1089/vbz.2010.0179. Epub 2010 Dec 13. Vector Borne Zoonotic Dis. 2011. PMID: 21142950 Free PMC article.
References
-
- Angsubhakorn, S., J. B. Moe, J. R. Latendresse, G. S. Ward, M. Ngamprochana, S. Sahaphong, and N. Bhamarapravati. 1986. The neurovirulence of flaviviruses in crab-eating monkeys (Macaca fascicularis). Southeast Asian J. Trop. Med. Public Health 17604-612. - PubMed
-
- Asher, D. M. 1975. Movement disorders in rhesus monkeys after infection with tick-borne encephalitis virus, vol. 10, p. 277-283. In B. S. Meldrum and C. D. Marsden (ed.), Advances in neurology. Raven Press, New York, NY. - PubMed
-
- Bodian, D., I. M. Morgan, and C. E. Schwerdt. 1950. Virus and host factors influencing the titer of Lansing poliomyelitis virus in monkeys, cotton rats and mice. Am. J. Hyg. 51126-133. - PubMed
-
- Durbin, A. P., R. A. Karron, W. Sun, D. W. Vaughn, M. J. Reynolds, J. R. Perreault, B. Thumar, R. Men, C. J. Lai, W. R. Elkins, R. M. Chanock, B. R. Murphy, and S. S. Whitehead. 2001. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in the 3′ untranslated region. Am. J. Trop. Med. Hyg. 65405-413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

