Structural basis of GM1 ganglioside recognition by simian virus 40
- PMID: 18353982
- PMCID: PMC2278218
- DOI: 10.1073/pnas.0710301105
Structural basis of GM1 ganglioside recognition by simian virus 40
Abstract
Simian virus 40 (SV40) has been a paradigm for understanding attachment and entry of nonenveloped viruses, viral DNA replication, and virus assembly, as well as for endocytosis pathways associated with caveolin and cholesterol. We find by glycan array screening that SV40 recognizes its ganglioside receptor GM1 with a quite narrow specificity, but isothermal titration calorimetry shows that individual binding sites have a relatively low affinity, with a millimolar dissociation constant. The high-resolution crystal structure of recombinantly produced SV40 capsid protein, VP1, in complex with the carbohydrate portion of GM1, reveals that the receptor is bound in a shallow solvent-exposed groove at the outer surface of the capsid. Through a complex network of interactions, VP1 recognizes a conformation of GM1 that is the dominant one in solution. Analysis of contacts provides a structural basis for the observed specificity and suggests binding mechanisms for additional physiologically relevant GM1 variants. Comparison with murine Polyomavirus (Polyoma) receptor complexes reveals that SV40 uses a different mechanism of sialic acid binding, which has implications for receptor binding of human polyomaviruses. The SV40-GM1 complex reveals a parallel to cholera toxin, which uses a similar cell entry pathway and binds GM1 in the same conformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
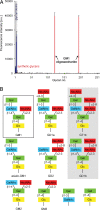


Comment in
-
A virus takes an "L" turn to find its receptor.Cell Host Microbe. 2010 Oct 21;8(4):301-2. doi: 10.1016/j.chom.2010.10.003. Cell Host Microbe. 2010. PMID: 20951962
References
-
- Eddy BE, Borman GS, Grubbs GE, Young RD. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology. 1962;17:65–75. - PubMed
-
- Girardi AJ, Sweet BH, Slotnick VB, Hilleman MR. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV-40. Proc Soc Exp Biol Med. 1962;109:649–660. - PubMed
-
- Berger JR, Major EO. Progressive multifocal leukoencephalopathy. Semin Neurol. 1999;19:193–200. - PubMed
-
- Drachenberg CB, et al. Human polyoma virus in renal allograft biopsies: morphological findings and correlation with urine cytology. Hum Pathol. 1999;30:970–977. - PubMed
-
- Stehle T, Gamblin SJ, Yan Y, Harrison SC. The structure of simian virus 40 refined at 3.1 Å resolution. Structure (London) 1996;4:165–182. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

