Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment
- PMID: 18354005
- PMCID: PMC6670720
- DOI: 10.1523/JNEUROSCI.4761-07.2008
Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment
Abstract
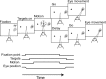
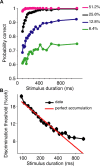
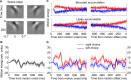
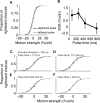
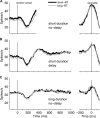
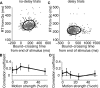
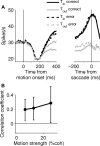
Decisions about sensory stimuli are often based on an accumulation of evidence in time. When subjects control stimulus duration, the decision terminates when the accumulated evidence reaches a criterion level. Under many natural circumstances and in many laboratory settings, the environment, rather than the subject, controls the stimulus duration. In these settings, it is generally assumed that subjects commit to a choice at the end of the stimulus stream. Indeed, failure to benefit from the full stream of information is interpreted as a sign of imperfect accumulation or memory leak. Contrary to these assumptions, we show that monkeys performing a direction discrimination task commit to a choice when the accumulated evidence reaches a threshold level (or bound), sometimes long before the end of stimulus. This bounded accumulation of evidence is reflected in the activity of neurons in the lateral intraparietal cortex. Thus, the readout of visual cortex embraces a termination rule to limit processing even when potentially useful information is available.
Figures

References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A. 1985;2:284–299. - PubMed
-
- Bogacz R. Optimal decision network with distributed representation. Neural Netw. 2007;20:564–576. - PubMed
-
- Bracewell RM, Mazzoni P, Barash S, Andersen RA. Motor intention activity in the macaque's lateral intraparietal area. II. Changes of motor plan. J Neurophysiol. 1996;76:1457–1464. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
