Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway
- PMID: 18354012
- PMCID: PMC2670451
- DOI: 10.1523/JNEUROSCI.5266-07.2008
Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway
Abstract
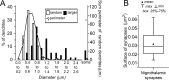
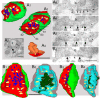
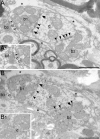
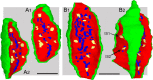
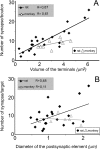
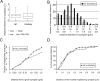
Giant inhibitory terminals with multiple synapses, the counterparts of excitatory "detonator" or "driver" terminals, have not been described in the forebrain. Using three-dimensional reconstructions of electron microscopic images, we quantitatively characterize a GABAergic pathway that establishes synaptic contacts exclusively via multiple synapses. Axon terminals of the nigrothalamic pathway formed, on average, 8.5 synapses on large-diameter dendrites and somata of relay cells in the ventromedial nucleus of the rat thalamus. All synapses of a given terminal converged on a single postsynaptic element. The vast majority of the synapses established by a single terminal were not separated by astrocytic processes. Nigrothalamic terminals in the macaque monkey showed the same ultrastructural features both in qualitative and quantitative terms (the median number of synapse per target was also 8.5). The individual synapses were closely spaced in both species. The nearest-neighbor synaptic distances were 169 nm in the rat and 178 nm in the monkey. The average number of synapses within 0.75 microm from any given synapse was 3.8 in the rat and 3.5 in the monkey. The arrangement of synapses described in this study creates favorable conditions for intersynaptic spillover of GABA among the multiple synapses of a single bouton, which can result in larger charge transfer. This could explain faithful and efficient GABAergic signal transmission in the nigrothalamic pathway in the healthy condition and during Parkinson's disease. In addition, our structural data suggest that the rodent nigrothalamic pathway can be a valid model of the primate condition, when the mechanism of GABAergic transmission is studied.
Figures



Similar articles
-
Contrasting the functional properties of GABAergic axon terminals with single and multiple synapses in the thalamus.J Neurosci. 2008 Nov 12;28(46):11848-61. doi: 10.1523/JNEUROSCI.3183-08.2008. J Neurosci. 2008. PMID: 19005050 Free PMC article.
-
Synapses on GABAergic neurons in the basolateral nucleus of the rat amygdala: double-labeling immunoelectron microscopy.Synapse. 2002 Jan;43(1):42-50. doi: 10.1002/syn.10017. Synapse. 2002. PMID: 11746732
-
Selective GABAergic innervation of thalamic nuclei from zona incerta.Eur J Neurosci. 2002 Sep;16(6):999-1014. doi: 10.1046/j.1460-9568.2002.02157.x. Eur J Neurosci. 2002. PMID: 12383229
-
Localization of GABA receptors in the basal ganglia.Prog Brain Res. 2007;160:229-43. doi: 10.1016/S0079-6123(06)60013-7. Prog Brain Res. 2007. PMID: 17499117 Review.
-
Emerging themes in GABAergic synapse development.Prog Neurobiol. 2011 Sep 15;95(1):68-87. doi: 10.1016/j.pneurobio.2011.07.002. Epub 2011 Jul 20. Prog Neurobiol. 2011. PMID: 21798307 Free PMC article. Review.
Cited by
-
Cell Type-Specific Decrease of the Intrinsic Excitability of Motor Cortical Pyramidal Neurons in Parkinsonism.J Neurosci. 2021 Jun 23;41(25):5553-5565. doi: 10.1523/JNEUROSCI.2694-20.2021. Epub 2021 May 18. J Neurosci. 2021. PMID: 34006589 Free PMC article.
-
Anatomical Development of the Cerebellothalamic Tract in Embryonic Mice.Cells. 2022 Nov 27;11(23):3800. doi: 10.3390/cells11233800. Cells. 2022. PMID: 36497060 Free PMC article.
-
Basal ganglia output to the thalamus: still a paradox.Trends Neurosci. 2013 Dec;36(12):695-705. doi: 10.1016/j.tins.2013.09.001. Epub 2013 Nov 2. Trends Neurosci. 2013. PMID: 24188636 Free PMC article. Review.
-
Basal ganglia-thalamus and the "crowning enigma".Front Neural Circuits. 2015 Nov 4;9:71. doi: 10.3389/fncir.2015.00071. eCollection 2015. Front Neural Circuits. 2015. PMID: 26582979 Free PMC article. Review.
-
Premotor Ramping of Thalamic Neuronal Activity Is Modulated by Nigral Inputs and Contributes to Control the Timing of Action Release.J Neurosci. 2021 Mar 3;41(9):1878-1891. doi: 10.1523/JNEUROSCI.1204-20.2020. Epub 2021 Jan 14. J Neurosci. 2021. PMID: 33446518 Free PMC article.
References
-
- Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983;286:237–265. - PubMed
-
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. - PubMed
-
- Bar-Gad I, Morris G, Bergman H. Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol. 2003;71:439–473. - PubMed
-
- Bartho P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
