Nonlinear integration of binocular optic flow by DNOVS2, a descending neuron of the fly
- PMID: 18354016
- PMCID: PMC6670693
- DOI: 10.1523/JNEUROSCI.5460-07.2008
Nonlinear integration of binocular optic flow by DNOVS2, a descending neuron of the fly
Abstract
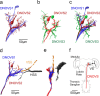
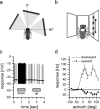
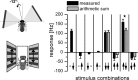
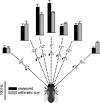
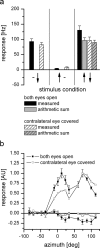
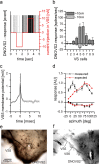
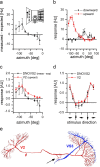
For visual orientation and course stabilization, flies rely heavily on the optic flow perceived by the animal during flight. The processing of optic flow is performed in motion-sensitive tangential cells of the lobula plate, which are well described with respect to their visual response properties and the connectivity among them. However, little is known about the postsynaptic descending neurons, which convey motion information to the motor circuits in the thoracic ganglion. Here we investigate the physiology and connectivity of an identified premotor descending neuron, called DNOVS2 (for descending neuron of the ocellar and vertical system). We find that DNOVS2 is tuned in a supralinear way to rotation around the longitudinal body axis. Experiments involving stimulation of the ipsilateral and the contralateral eye indicate that ipsilateral computation of motion information is modified nonlinearly by motion information from the contralateral eye. Performing double recordings of DNOVS2 and lobula plate tangential cells, we find that DNOVS2 is connected ipsilaterally to a subset of vertical-sensitive cells. From the contralateral eye, DNOVS2 receives input most likely from V2, a heterolateral spiking neuron. This specific neural circuit is sufficient for the tuning of DNOVS2, making it probably an important element in optomotor roll movements of the head and body around the fly's longitudinal axis.
Figures
References
-
- Beersma DGM, Stavenga DG, Kuiper JW. Retinal lattice, visual field and binocularities in flies. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1977;119:207–220.
-
- Borst A. Fly visual interneurons responsive to image expansion. Zool Jb Physiol. 1991;95:305–313.
-
- Borst A, Bahde S. Spatio-temporal integration of motion: a simple strategy for safe landing in flies. Naturwissenschaften. 1988;75:265–267.
-
- Borst A, Haag J. Neural networks in the cockpit of the fly. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:419–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
