The fission yeast homologue of CENP-B, Abp1, regulates directionality of mating-type switching
- PMID: 18354497
- PMCID: PMC2323252
- DOI: 10.1038/emboj.2008.53
The fission yeast homologue of CENP-B, Abp1, regulates directionality of mating-type switching
Abstract
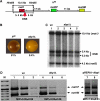
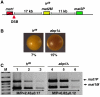
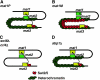
In fission yeast, mating-type switching involves replacing genetic information contained at the expressed mat1 locus by that of either the mat2P or mat3M donor loci. Donor selection is nonrandom, as mat1P cells preferentially use mat3M for switching, whereas mat1M cells use mat2P. Switching directionality is determined by the cell-type-specific distribution of the Swi2-Swi5 complex that, in mat1P cells, localises to mat3M and, only in mat1M cells, spreads to mat2P in a heterochromatin-dependent manner. Mechanisms regulating spreading of Swi2-Swi5 across heterochromatin are not fully understood. Here, we show that the fission yeast homologue of CENP-B, Abp1, binds to the silent domain of the mating-type locus and regulates directionality of switching. Deletion of abp1 prevents utilisation of mat2P, as when heterochromatin is disrupted and spreading of Swi2-Swi5 is impaired. Our results show that, indeed, deletion of abp1 abolishes spreading of Swi2-Swi5 to mat2P. However, in abp1Delta cells, heterochromatin organisation at the mating-type locus is preserved, indicating that Abp1 is actually required for efficient spreading of Swi2-Swi5 through heterochromatin. Cbh1 and Cbh2, which are also homologous to CENP-B, have only a minor contribution to the regulation of directionality of switching, which is in contrast with the strong effects observed for Abp1.
Figures



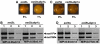

References
-
- Arcangioli B, Thon G (2004) Mating-type cassettes: structure, switching and silencing. In The Molecular Biology of Schizosaccharomyces pombe, Egel R (ed), pp 129–144. Berlin, Heidelberg, New York: Springer-Verlag
-
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

