Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe-2S] clusters
- PMID: 18354500
- PMCID: PMC2323258
- DOI: 10.1038/emboj.2008.50
Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe-2S] clusters
Abstract
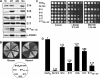
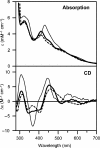
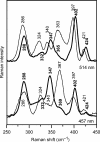
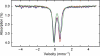
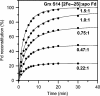
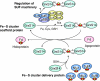
Glutaredoxins (Grxs) are small oxidoreductases that reduce disulphide bonds or protein-glutathione mixed disulphides. More than 30 distinct grx genes are expressed in higher plants, but little is currently known concerning their functional diversity. This study presents biochemical and spectroscopic evidence for incorporation of a [2Fe-2S] cluster in two heterologously expressed chloroplastic Grxs, GrxS14 and GrxS16, and in vitro cysteine desulphurase-mediated assembly of an identical [2Fe-2S] cluster in apo-GrxS14. These Grxs possess the same monothiol CGFS active site as yeast Grx5 and both were able to complement a yeast grx5 mutant defective in Fe-S cluster assembly. In vitro kinetic studies monitored by CD spectroscopy indicate that [2Fe-2S] clusters on GrxS14 are rapidly and quantitatively transferred to apo chloroplast ferredoxin. These data demonstrate that chloroplast CGFS Grxs have the potential to function as scaffold proteins for the assembly of [2Fe-2S] clusters that can be transferred intact to physiologically relevant acceptor proteins. Alternatively, they may function in the storage and/or delivery of preformed Fe-S clusters or in the regulation of the chloroplastic Fe-S cluster assembly machinery.
Figures



References
-
- Achebach S, Tran QH, Vlamis-Gardikas A, Mullner M, Holmgren A, Unden G (2004) Stimulation of Fe–S cluster insertion into apoFNR by E. coli glutaredoxins 1, 2 and 3 in vitro. FEBS Lett 565: 203–206 - PubMed
-
- Agar JN, Krebs B, Frazzon J, Huynh BH, Dean DR, Johnson MK (2000) IscU as a scaffold for iron–sulfur cluster biosynthesis: sequential assembly of [2Fe–2S] and [4Fe–4S] clusters in IscU. Biochemistry 39: 7856–7862 - PubMed
-
- Balk J, Lobreaux S (2005) Biogenesis of iron–sulfur proteins in plants. Trends Plant Sci 10: 324–331 - PubMed
-
- Belli G, Polaina J, Tamarit J, De La Torre MA, Rodriguez-Manzaneque MT, Ros J, Herrero E (2002) Structure–function analysis of yeast Grx5 monothiol glutaredoxin defines essential amino acids for the function of the protein. J Biol Chem 277: 37590–37596 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

