Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas
- PMID: 18356283
- PMCID: PMC2613818
- DOI: 10.1215/15228517-2007-062
Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas
Abstract
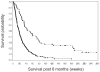
The North American Brain Tumor Consortium (NABTC) uses 6-month progression-free survival (6moPFS) as the efficacy end point of therapy trials for adult patients with recurrent high-grade gliomas. In this study, we investigated whether progression status at 6 months predicts survival from that time, implying the potential for prolonged survival if progression could be delayed. We also evaluated earlier time points to determine whether the time of progression assessment alters the strength of the prediction. Data were from 596 patient enrollments (159 with grade III gliomas and 437 with grade IV tumors) in NABTC phase II protocols between February 1998 and December 2002. Outcome was assessed statistically using Kaplan-Meier curves and Cox proportional hazards models. Median survivals were 39 and 30 weeks for patients with grade III and grade IV tumors, respectively. Twenty-eight percent of patients with grade III and 16% of patients with grade IV tumors had progression-free survival of >26 weeks. Progression status at 9, 18, and 26 weeks predicted survival from those times for patients with grade III or grade IV tumors (p < 0.001 and hazard ratios < 0.5 in all cases). Including KPS, age, number of prior chemotherapies, and response in a multivariate model did not substantively change the results. Progression status at 6 months is a strong predictor of survival, and 6moPFS is a valid end point for trials of therapy for recurrent malignant glioma. Earlier assessments of progression status also predicted survival and may be incorporated in the design of future clinical trials.
Figures

Comment in
-
In reference to lamborn et Al. (Neuro-oncology. 2008;10:162-170).Neuro Oncol. 2008 Dec;10(6):1171-2. doi: 10.1215/15228517-2008-055. Epub 2008 Aug 25. Neuro Oncol. 2008. PMID: 18725461 Free PMC article. No abstract available.
References
-
- U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Food and Drug Administration. FDA Project on Cancer Drug Approval Endpoints. [Accessed January 10, 2008]. Last updated April 20, 2007. Available at http://www.fda.gov/cder/drug/cancer_endpoints.
-
- U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Food and Drug Administration. Guidance for Industry, Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. 2007. [Accessed January 10, 2008]. Available at http://www.fda.gov/cder/guidance/7478fnl.pdf.
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. - PubMed
-
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- U01 CA 62399 022030/CA/NCI NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- U01 CA062399/CA/NCI NIH HHS/United States
- U01 CA 62407-08/CA/NCI NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- CA 62455-08/CA/NCI NIH HHS/United States
- CA 62426/CA/NCI NIH HHS/United States
- M01 RR 00042/RR/NCRR NIH HHS/United States
- CA 62422/CA/NCI NIH HHS/United States
- U01 CA062407/CA/NCI NIH HHS/United States
- U01 CA062426/CA/NCI NIH HHS/United States
- M01 RR000079/RR/NCRR NIH HHS/United States
- M01 RR 00633/RR/NCRR NIH HHS/United States
- M01 RR003186/RR/NCRR NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- U01 CA 62405/CA/NCI NIH HHS/United States
- M01 RR 00079/RR/NCRR NIH HHS/United States
- CA 62412/CA/NCI NIH HHS/United States
- M01 RR000633/RR/NCRR NIH HHS/United States
- U01 CA062405/CA/NCI NIH HHS/United States
- U01 CA062412/CA/NCI NIH HHS/United States
- M01 RR 0865/RR/NCRR NIH HHS/United States
- M01 RR 00056/RR/NCRR NIH HHS/United States
- CA 16672/CA/NCI NIH HHS/United States
- CA 62399/CA/NCI NIH HHS/United States
- U01 CA 62421-08/CA/NCI NIH HHS/United States
- U01 CA062421/CA/NCI NIH HHS/United States
- U01 CA062422/CA/NCI NIH HHS/United States
- M01 RR 03186/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

