Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits
- PMID: 18356479
- PMCID: PMC2494830
- DOI: 10.1152/japplphysiol.01345.2007
Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits
Abstract
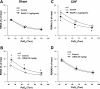
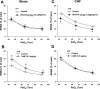
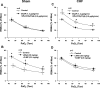
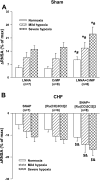
Peripheral chemoreflex sensitivity is potentiated in clinical and experimental chronic heart failure (CHF). Downregulation of nitric oxide (NO) synthase (NOS) in the carotid body (CB) is involved in this effect. However, it remains poorly understood whether carbon monoxide (CO) also contributes to the altered peripheral chemoreflex sensitivity in CHF. This work highlights the effect of NO and CO on renal sympathetic nerve activity (RSNA) in response to graded hypoxia in conscious rabbits. Renal sympathetic nerve responses to graded hypoxia were enhanced in CHF rabbits compared with sham rabbits. The NO donor S-nitroso-N-acetylpenicillamine (SNAP, 1.2 microg x kg(-1) x min(-1)) and the CO-releasing molecule tricarbonyldichlororuthenium (II) dimer {[Ru(CO)(3)Cl(2)](2), 3.0 microg x kg(-1) x min(-1)} each attenuated hypoxia-induced RSNA increases in CHF rabbits (P < 0.05), but the degree of attenuation of RSNA induced by SNAP or [Ru(CO)(3)Cl(2)](2) was smaller than that induced by SNAP + [Ru(CO)(3)Cl(2)](2). Conversely, treatment with the NOS inhibitor N(omega)-nitro-L-arginine (30 mg/kg) + the heme oxygenase (HO) inhibitor Cr (III) mesoporphyrin IX chloride (0.5 mg/kg) augmented the renal sympathetic nerve response to hypoxia in sham rabbits to a greater extent than treatment with either inhibitor alone and was without effect in CHF rabbits. In addition, using immunostaining and Western blot analyses, we found that expression of neuronal NOS, endothelial NOS, and HO-2 protein (expressed as the ratio of NOS or HO-2 expression to beta-tubulin protein expression) was lower in CBs from CHF (0.19 +/- 0.04, 0.17 +/- 0.06, and 0.15 +/- 0.02, respectively) than sham (0.63 +/- 0.04, 0.56 +/- 0.06, and 0.27 +/- 0.03, respectively) rabbits (P < 0.05). These results suggest that a deficiency of NO and CO in the CBs augments peripheral chemoreflex sensitivity to hypoxia in CHF.
Figures

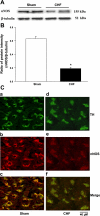
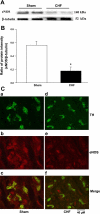
Comment in
-
NO and CO have got to GO for enhanced chemoreceptor sympathoexcitation in heart failure.J Appl Physiol (1985). 2008 Jul;105(1):1-2. doi: 10.1152/japplphysiol.90569.2008. Epub 2008 May 1. J Appl Physiol (1985). 2008. PMID: 18450975 No abstract available.
References
-
- Bundock EA, Drummond GS, Kappas A. Tissue distribution of synthetic heme analogues: studies with tin, chromium, and zinc mesoporphyrins. Pharmacology 52: 187–198, 1996. - PubMed
-
- Chua TP, Ponikowski P, Webb-Peploe K, Harrington D, Anker SD, Piepoli M, Coats AJ. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur Heart J 18: 480–486, 1997. - PubMed
-
- Chugh SS, Chua TP, Coats AJS. Peripheral chemoreflex in chronic heart failure: friend and foe. Am Heart J 132: 900–904, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

