Neonatal bone marrow transplantation of ADA-deficient SCID mice results in immunologic reconstitution despite low levels of engraftment and an absence of selective donor T lymphoid expansion
- PMID: 18356486
- PMCID: PMC2424166
- DOI: 10.1182/blood-2007-08-103663
Neonatal bone marrow transplantation of ADA-deficient SCID mice results in immunologic reconstitution despite low levels of engraftment and an absence of selective donor T lymphoid expansion
Abstract
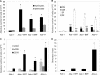
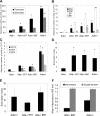
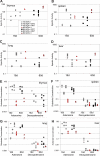
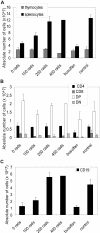
Adenosine deaminase (ADA)-deficient severe combined immune deficiency (SCID) may be treated by allogeneic hematopoietic stem cell transplantation without prior cytoreductive conditioning, although the mechanism of immune reconstitution is unclear. We studied this process in a murine gene knockout model of ADA-deficient SCID. Newborn ADA-deficient pups received transplants of intravenous infusion of normal congenic bone marrow, without prior cytoreductive conditioning, which resulted in long-term survival, multisystem correction, and nearly normal lymphocyte numbers and mitogenic proliferative responses. Only 1% to 3% of lymphocytes and myeloid cells were of donor origin without a selective expansion of donor-derived lymphocytes; immune reconstitution was by endogenous, host-derived ADA-deficient lymphocytes. Preconditioning of neonates with 100 to 400 cGy of total body irradiation before normal donor marrow transplant increased the levels of engrafted donor cells in a radiation dose-dependent manner, but the chimerism levels were similar for lymphoid and myeloid cells. The absence of selective reconstitution by donor T lymphocytes in the ADA-deficient mice indicates that restoration of immune function occurred by rescue of endogenous ADA-deficient lymphocytes through cross-correction from the engrafted ADA-replete donor cells. Thus, ADA-deficient SCID is unique in its responses to nonmyeloablative bone marrow transplantation, which has implications for clinical bone marrow transplantation or gene therapy.
Figures



References
-
- Hershfield M S, Mitchell B S. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York, NY: McGraw-Hill; 2001. pp. 2585–2625.
-
- Small T N, Freidrich W, O'Reilly R J. Hematopoietic stem cell transplantation for immunodeficiency diseases. In: Blume KC, Forman SJ, Appelbaum FR, editors. Hematopoietic Stem Cell Transplantation. 3rd ed. Malden, MA: Blackwell Science; 2004. pp. 1430–1442.
-
- Buckley RH, Schiff SE, Schiff RI, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. - PubMed
-
- van Leeuwen JE, van Tol MJ, Joosten AM, et al. Relationship between patterns of engraftment in peripheral blood and immune reconstitution after allogeneic bone marrow transplantation for (severe) combined immunodeficiency. Blood. 1994;84:3936–3947. - PubMed
-
- Tjonnfjord GE, Steen R, Veiby OP, Friedrich W, Egeland T. Evidence for engraftment of donor-type multipotent CD34+ cells in a patient with selective T-lymphocyte reconstitution after bone marrow transplantation for B-SCID. Blood. 1994;84:3584–3589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

