Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation
- PMID: 18356542
- PMCID: PMC2629616
- DOI: 10.1161/CIRCRESAHA.107.167072
Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation
Abstract
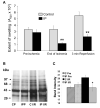
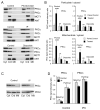
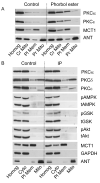
Inhibition of mitochondrial permeability transition pore (MPTP) opening at reperfusion is critical for cardioprotection by ischemic preconditioning (IP). Some studies have implicated mitochondrial protein phosphorylation in this effect. Here we confirm that mitochondria rapidly isolated from preischemic control and IP hearts show no significant difference in calcium-mediated MPTP opening, whereas IP inhibits MPTP opening in mitochondria isolated from IP hearts following 30 minutes of global normothermic ischemia or 3 minutes of reperfusion. Analysis of protein phosphorylation in density-gradient purified mitochondria was performed using both 2D and 1D electrophoresis, with detection of phosphoproteins using Pro-Q Diamond or phospho-amino-specific antibodies. Several phosphoproteins were detected, including voltage-dependent anion channels isoforms 1 and 2, but none showed significant IP-mediated changes either before ischemia or during ischemia and reperfusion, and neither Western blotting nor 2D fluorescence difference gel electrophoresis detected translocation of protein kinase C (alpha, epsilon, or delta isoforms), glycogen synthase kinase 3beta, or Akt to the mitochondria following IP. In freeze-clamped hearts, changes in phosphorylation of GSK3beta, Akt, and AMP-activated protein kinase were detected following ischemia and reperfusion but no IP-mediated changes correlated with MPTP inhibition or cardioprotection. However, measurement of mitochondrial protein carbonylation, a surrogate marker for oxidative stress, suggested that a reduction in mitochondrial oxidative stress at the end of ischemia and during reperfusion may account for IP-mediated inhibition of MPTP. The signaling pathways mediating this effect and maintaining it during reperfusion are discussed.
Figures





References
-
- Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion - a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. - PubMed
-
- Yellon DM, Downey JM. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. - PubMed
-
- Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–122. - PubMed
-
- Khaliulin I, Schwalb H, Wang P, Houminer E, Grinberg L, Katzeff H, Borman JB, Powell SR. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radic Biol Med. 2004;37:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

