The neuronal Kv4 channel complex
- PMID: 18357523
- PMCID: PMC5833991
- DOI: 10.1007/s11064-008-9650-8
The neuronal Kv4 channel complex
Abstract
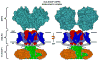
Kv4 channel complexes mediate the neuronal somatodendritic A-type K(+) current (I(SA)), which plays pivotal roles in dendritic signal integration. These complexes are composed of pore-forming voltage-gated alpha-subunits (Shal/Kv4) and at least two classes of auxiliary beta-subunits: KChIPs (K(+)-Channel-Interacting-Proteins) and DPLPs (Dipeptidyl-Peptidase-Like-Proteins). Here, we review our investigations of Kv4 gating mechanisms and functional remodeling by specific auxiliary beta-subunits. Namely, we have concluded that: (1) the Kv4 channel complex employs novel alternative mechanisms of closed-state inactivation; (2) the intracellular Zn(2+) site in the T1 domain undergoes a conformational change tightly coupled to voltage-dependent gating and is targeted by nitrosative modulation; and (3) discrete and specific interactions mediate the effects of KChIPs and DPLPs on activation, inactivation and permeation of Kv4 channels. These studies are shedding new light on the molecular bases of I(SA) function and regulation.
Figures
References
-
- Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. - PubMed
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Bahring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J Biol Chem. 2001;276:23888–23894. - PubMed
-
- Baldwin TJ, Tsaur ML, Lopez GA, Jan YN, Jan LY. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

