Epigenetics and its implications for behavioral neuroendocrinology
- PMID: 18358518
- PMCID: PMC2394853
- DOI: 10.1016/j.yfrne.2008.01.003
Epigenetics and its implications for behavioral neuroendocrinology
Abstract
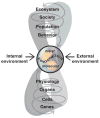
Individuals vary in their sociosexual behaviors and reactivity. How the organism interacts with the environment to produce this variation has been a focus in psychology since its inception as a scientific discipline. There is now no question that cumulative experiences throughout life history interact with genetic predispositions to shape the individual's behavior. Recent evidence suggests that events in past generations may also influence how an individual responds to events in their own life history. Epigenetics is the study of how the environment can affect the genome of the individual during its development as well as the development of its descendants, all without changing the DNA sequence. Several distinctions must be made if this research is to become a staple in behavioral neuroendocrinology. The first distinction concerns perspective, and the need to distinguish and appreciate, the differences between Molecular versus Molar epigenetics. Each has its own lineage of investigation, yet both appear to be unaware of one another. Second, it is important to distinguish the difference between Context-Dependent versus Germline-Dependent epigenetic modifications. In essence the difference is one of the mechanism of heritability or transmission within, as apposed to across, generations. This review illustrates these distinctions while describing several rodent models that have shown particular promise for unraveling the contribution of genetics and the environment on sociosexual behavior. The first focuses on genetically-modified mice and makes the point that the early litter environment alters subsequent brain activity and behavior. This work emphasizes the need to understand behavioral development when doing research with such animals. The second focuses on a new rat model in which the epigenome is permanently imprinted, an effect that crosses generations to impact the descendants without further exposure to the precipitating agent. This work raises the question of how events in generations past can have consequences at both the mechanistic, behavioral, and ultimately evolutionary levels.
Figures





References
-
- Ames N, Gilley J, Fried M. The comparative genomic structure and sequence of the surfeit gene homologs in the puffer fish Fugu rubripes and their association with CpG-rich islands. Genome Res. 1997;7:1138–1152. - PubMed
-
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147(Suppl):S43–S49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

