PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B
- PMID: 18358807
- PMCID: PMC2394663
- DOI: 10.1016/j.cell.2008.01.043
PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B
Abstract
Glycoprotein B (gB) is one of the essential components for infection by herpes simplex virus-1 (HSV-1). Although several cellular receptors that associate with glycoprotein D (gD), such as herpes virus entry mediator (HVEM) and Nectin-1, have been identified, specific molecules that mediate HSV-1 infection by associating with gB have not been elucidated. Here, we found that paired immunoglobulin-like type 2 receptor (PILR) alpha associates with gB, and cells transduced with PILRalpha become susceptible to HSV-1 infection. Furthermore, HSV-1 infection of human primary cells expressing both HVEM and PILRalpha was blocked by either anti-PILRalpha or anti-HVEM antibody. Our results demonstrate that cellular receptors for both gB and gD are required for HSV-1 infection and that PILRalpha plays an important role in HSV-1 infection as a coreceptor that associates with gB. These findings uncover a crucial aspect of the mechanism underlying HSV-1 infection.
Figures

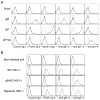




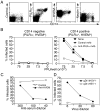
References
-
- Chapman TL, You I, Joseph IM, Bjorkman PJ, Morrison SL, Raghavan M. Characterization of the interaction between the herpes simplex virus type I Fc receptor and immunoglobulin G. J Biol Chem. 1999;274:6911–6919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

