Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly
- PMID: 18358808
- PMCID: PMC2291539
- DOI: 10.1016/j.cell.2008.01.019
Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly
Abstract
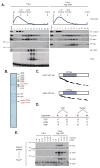
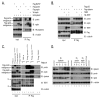
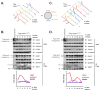
Telomerase is a multisubunit ribonucleoprotein (RNP) complex that adds telomere repeats to the ends of chromosomes. Three essential telomerase components have been identified thus far: the telomerase reverse transcriptase (TERT), the telomerase RNA component (TERC), and the TERC-binding protein dyskerin. Few other proteins are known to be required for human telomerase function, limiting our understanding of both telomerase regulation and mechanisms of telomerase action. Here, we identify the ATPases pontin and reptin as telomerase components through affinity purification of TERT from human cells. Pontin interacts directly with both TERT and dyskerin, and the amount of TERT bound to pontin and reptin peaks in S phase, evidence for cell-cycle-dependent regulation of TERT. Depletion of pontin and reptin markedly impairs telomerase RNP accumulation, indicating an essential role in telomerase assembly. These findings reveal an unanticipated requirement for additional enzymes in telomerase biogenesis and suggest alternative approaches for inhibiting telomerase in cancer.
Figures




References
-
- Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. - PubMed
-
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–15964. - PMC - PubMed
-
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. - PubMed
-
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. - PubMed
-
- Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

