Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration
- PMID: 18359072
- PMCID: PMC2574644
- DOI: 10.1016/j.dental.2008.02.001
Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration
Abstract
Objectives: Seven million people suffer bone fractures each year in the U.S., and musculoskeletal conditions cost $215 billion/year. The objectives of this study were to develop moldable/injectable, mechanically strong and in situ-hardening calcium phosphate cement (CPC) composite scaffolds for bone regeneration and delivery of osteogenic cells and growth factors.
Methods: Tetracalcium phosphate [TTCP: Ca(4)(PO(4))(2)O] and dicalcium phosphate (DCPA: CaHPO(4)) were used to fabricate self-setting calcium phosphate cement. Strong and macroporous scaffolds were developed via absorbable fibers, biopolymer chitosan, and mannitol porogen. Following established protocols, MC3T3-E1 osteoblast-like cells (Riken, Hirosaka, Japan) were cultured on the specimens and inside the CPC composite paste carrier.
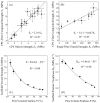
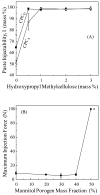
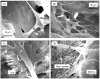
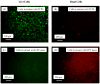
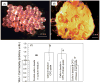
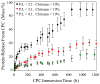
Results: The scaffold strength was more than doubled via reinforcement (p<0.05). Relationships and predictive models were established between matrix properties, fibers, porosity, and overall composite properties. The cement injectability was increased from about 60% to nearly 100%. Cell attachment and proliferation on the new composite matched those of biocompatible controls. Cells were able to infiltrate into the macropores and anchor to the bone mineral-like nano-apatite crystals. For cell delivery, alginate hydrogel beads protected cells during cement mixing and setting, yielding cell viability measured via the Wst-1 assay that matched the control without CPC (p>0.1). For growth factor delivery, CPC powder:liquid ratio and chitosan content provided the means to tailor the rate of protein release from CPC carrier.
Significance: New CPC scaffolds were developed that were strong, tough, macroporous and osteoconductive. They showed promise for injection in minimally invasive surgeries, and in delivering osteogenic cells and osteoinductive growth factors to promote bone regeneration. Potential applications include various dental, craniofacial, and orthopedic reconstructions.
Figures


References
-
- Praemer A, Furner S, Rice DP. Musculoskeletal conditions in the United States. Rosemont: Amer Acad Orthop Surg; 1999.
-
- Franceshi RT. Biological approaches to bone regeneration by gene therapy. Critical Reviews in Oral Bio Med. 2005;84:1093–1103. - PubMed
-
- Medical Data International. RP-651147 - Orthopedic and musculoskeletal markets: Biotechnology and tissue engineering. 2000. pp. 28–31.
-
- Ambrosio AMA, Sahota JS, Khan Y, Laurencin CT. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J Biomed Mater Res. 2001;58B:295–301. - PubMed
-
- Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA. Tissue engineering: Orthopedic applications. Annual Rev Biomed Eng. 1999;1:19–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

