Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI
- PMID: 18359777
- PMCID: PMC2583155
- DOI: 10.1093/cercor/bhn036
Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI
Abstract
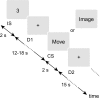
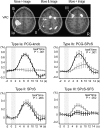
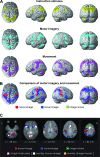
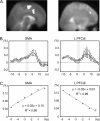
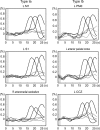
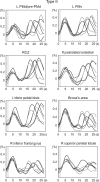
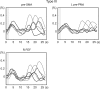
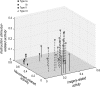
Activation of motor-related areas has consistently been found during various motor imagery tasks and is regarded as the central mechanism generating motor imagery. However, the extent to which motor execution and imagery share neural substrates remains controversial. We examined brain activity during preparation for and execution of physical or mental finger tapping. During a functional magnetic resonance imaging at 3 T, 13 healthy volunteers performed an instructed delay finger-tapping task either in a physical mode or mental mode. Number stimuli instructed subjects about a finger-tapping sequence. After an instructed delay period, cue stimuli prompted them either to execute the tapping movement or to imagine it. Two types of planning/preparatory activity common for movement and imagery were found: instruction stimulus-related activity represented widely in multiple motor-related areas and delay period activity in the medial frontal areas. Although brain activity during movement execution and imagery was largely shared in the distributed motor network, imagery-related activity was in general more closely related to instruction-related activity than to the motor execution-related activity. Specifically, activity in the medial superior frontal gyrus, anterior cingulate cortex, precentral sulcus, supramarginal gyrus, fusiform gyrus, and posterolateral cerebellum likely reflects willed generation of virtual motor commands and analysis of virtual sensory signals.
Figures
References
-
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. - PubMed

