Part II: diffraction from two-dimensional cholera toxin crystals bound to their receptors in a lipid monolayer
- PMID: 18359801
- PMCID: PMC2440462
- DOI: 10.1529/biophysj.107.120808
Part II: diffraction from two-dimensional cholera toxin crystals bound to their receptors in a lipid monolayer
Abstract
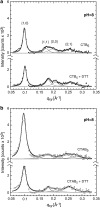
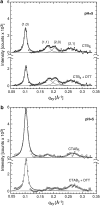
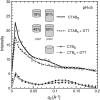
The structure of cholera toxin (CTAB(5)) bound to its putative ganglioside receptor, galactosyl-N-acetylgalactosaminyl (N-acetyl-neuraminyl) galactosylglucosylceramide (GM(1)), in a lipid monolayer at the air-water interface has been studied utilizing grazing incidence x-ray diffraction. Cholera toxin is one of very few proteins to be crystallized in two dimensions and characterized in a fully hydrated state. The observed grazing incidence x-ray diffraction Bragg peaks indicated cholera toxin was ordered in a hexagonal lattice and the order extended 600-800 A. The pentameric binding portion of cholera toxin (CTB(5)) improved in-plane ordering over the full toxin (CTAB(5)) especially at low pH. Disulfide bond reduction (activation of the full toxin) also increased the protein layer ordering. These findings are consistent with A-subunit flexibility and motion, which cause packing inefficiencies and greater disorder of the protein layer. Corroborative out-of-plane diffraction (Bragg rod) analysis indicated that the scattering units in the cholera layer with CTAB(5) shortened after disulfide bond reduction of the A subunit. These studies, together with Part I results, revealed key changes in the structure of the cholera toxin-lipid system under different pH conditions.
Figures

References
-
- Lenne, P. F., B. Berge, A. Renault, C. Zakri, C. Venien-Bryan, S. Courty, F. Balavoine, W. Bergsma-Schutter, A. Brisson, G. Grubel, N. Boudet, O. Konovalov, and J. F. Legrand. 2000. Synchrotron radiation diffraction from two-dimensional protein crystals at the air/water interface. Biophys. J. 79:496–500. - PMC - PubMed
-
- Verclas, S. A. W., P. A. Howes, K. Kjaer, A. Wurlitzer, M. Weygand, G. Buldt, N. A. Dencher, and M. Losche. 1999. X-ray diffraction from a single layer of purple membrane at the air/water interface. J. Mol. Biol. 287:837–843. - PubMed
-
- Verclas, S. A. W., P. A. Howes, K. Kjaer, A. Wurlitzer, M. Weygand, G. Buldt, N. A. Dencher, and M. Losche. 1999. X-ray diffraction from a single layer of purple membrane at the air/water interface. J. Mol. Biol. 287:837–843. - PubMed
-
- Weygand, M., K. Kjaer, P. B. Howes, B. Wetzer, D. Pum, U. B. Sleytr, and M. Losche. 2002. Structural reorganization of phospholipid headgroups upon recrystallization of an S-layer lattice. J. Phys. Chem. B. 106:5793–5799.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

