Part I: an x-ray scattering study of cholera toxin penetration and induced phase transformations in lipid membranes
- PMID: 18359802
- PMCID: PMC2440476
- DOI: 10.1529/biophysj.107.120725
Part I: an x-ray scattering study of cholera toxin penetration and induced phase transformations in lipid membranes
Abstract
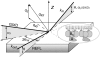
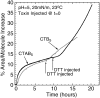
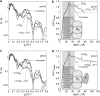
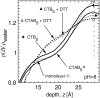
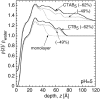
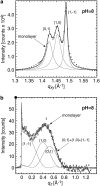
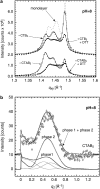
Cholera toxin is a highly efficient biotoxin, which is frequently used as a tool to investigate protein-membrane interactions and as a reporter for membrane rafts. Cholera toxin binds selectively to gangliosides with highest affinity to GM(1). However, the mechanism by which cholera toxin crosses the membrane remains unresolved. Using x-ray reflectivity and grazing incidence diffraction, we have been able to monitor the binding and penetration of cholera toxin into a model lipid monolayer containing the receptor GM(1) at the air-water interface. Very high toxin coverage was obtained allowing precise measurements of how toxin binding alters lipid packing. Grazing incidence x-ray diffraction revealed the coexistence of two monolayer phases after toxin binding. The first was identical to the monolayer before toxin binding. In regions where toxin was bound, a second membrane phase exhibited a decrease in order as evidenced by a larger area per molecule and tilt angle with concomitant thinning of the monolayer. These results demonstrate that cholera toxin binding induces the formation of structurally distinct, less ordered domains in gel phases. Furthermore, the largest decrease in lateral order to the monolayer occurred at low pH, supporting a low endosomal pH in the infection pathway. Surprisingly, at pH = 8 toxin penetration by the binding portion of the toxin, the B(5) pentamer, was also observed.
Figures


References
-
- Zhang, R. G., D. L. Scott, M. L. Westbrook, S. Nance, B. D. Spangler, G. G. Shipley, and E. M. Westbrook. 1995. The three-dimensional crystal structure of cholera toxin. J. Mol. Biol. 251:563–573. - PubMed
-
- Zhang, R. G., M. L. Westbrook, E. M. Westbrook, D. L. Scott, Z. Otwinowski, P. R. Maulik, R. A. Reed, and G. G. Shipley. 1995. The 2.4 angstrom crystal structure of cholera toxin B subunit pentamer—choleragenoid. J. Mol. Biol. 251:550–562. - PubMed
-
- Feng, Y., A. P. Jadhav, C. Rodighiero, Y. Fujinaga, T. Kirchhausen, and W. I. Lencer. 2004. Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans-Golgi network but not the Golgi apparatus in Exo2-treated cells. EMBO Rep. 5:596–601. - PMC - PubMed
-
- Mekalanos, J. J., R. J. Collier, and W. R. Romig. 1979. Enzymic activity of cholera toxin. 2. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J. Biol. Chem. 254:5855–5861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

