Concentration of enteroviruses, adenoviruses, and noroviruses from drinking water by use of glass wool filters
- PMID: 18359827
- PMCID: PMC2394941
- DOI: 10.1128/AEM.02246-07
Concentration of enteroviruses, adenoviruses, and noroviruses from drinking water by use of glass wool filters
Abstract
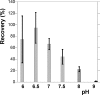
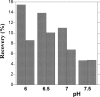
Available filtration methods to concentrate waterborne viruses are either too costly for studies requiring large numbers of samples, limited to small sample volumes, or not very portable for routine field applications. Sodocalcic glass wool filtration is a cost-effective and easy-to-use method to retain viruses, but its efficiency and reliability are not adequately understood. This study evaluated glass wool filter performance to concentrate the four viruses on the U.S. Environmental Protection Agency contaminant candidate list, i.e., coxsackievirus, echovirus, norovirus, and adenovirus, as well as poliovirus. Total virus numbers recovered were measured by quantitative reverse transcription-PCR (qRT-PCR); infectious polioviruses were quantified by integrated cell culture (ICC)-qRT-PCR. Recovery efficiencies averaged 70% for poliovirus, 14% for coxsackievirus B5, 19% for echovirus 18, 21% for adenovirus 41, and 29% for norovirus. Virus strain and water matrix affected recovery, with significant interaction between the two variables. Optimal recovery was obtained at pH 6.5. No evidence was found that water volume, filtration rate, and number of viruses seeded influenced recovery. The method was successful in detecting indigenous viruses in municipal wells in Wisconsin. Long-term continuous filtration retained viruses sufficiently for their detection for up to 16 days after seeding for qRT-PCR and up to 30 days for ICC-qRT-PCR. Glass wool filtration is suitable for large-volume samples (1,000 liters) collected at high filtration rates (4 liters min(-1)), and its low cost makes it advantageous for studies requiring large numbers of samples.
Figures
References
-
- American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
-
- Bosch, A., R. M. Pinto, A. R. Blanch, and J. T. Jofre. 1988. Detection of human rotavirus in sewage through two concentration procedures. Water Res. 22:343-348.
-
- Chapron, C. D., N. A. Ballester, J. H. Fontaine, C. N. Frades, and A. B. Margolin. 2000. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl. Environ. Microbiol. 66:2520-2525. - PMC - PubMed
-
- Cromeans, T., J. Narayanan, K. Jung, G. Ko, D. Wait, and M. Sobsey. 2005. Development of molecular methods to detect infectious viruses in water. Report no. 90995F. American Water Works Association Research Foundation (AwwaRF), Denver, CO.
-
- De Leon, R., C. Shieh, R. S. Baric, and M. D. Sobsey. 1990. Detection of enterovirus and hepatitis A virus in environmental samples by gene probes and polymerase chain reaction, p. 833-853. In Advances of water analysis and treatment proceedings 1989. Water Quality Technology Conference, American Water Works Association, Denver, CO. American Water Works Association, San Diego, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

