Occurrence and expression of gene transfer agent genes in marine bacterioplankton
- PMID: 18359833
- PMCID: PMC2394915
- DOI: 10.1128/AEM.02129-07
Occurrence and expression of gene transfer agent genes in marine bacterioplankton
Abstract
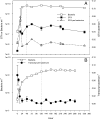
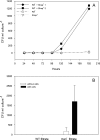
Genes with homology to the transduction-like gene transfer agent (GTA) were observed in genome sequences of three cultured members of the marine Roseobacter clade. A broader search for homologs for this host-controlled virus-like gene transfer system identified likely GTA systems in cultured Alphaproteobacteria, and particularly in marine bacterioplankton representatives. Expression of GTA genes and extracellular release of GTA particles ( approximately 50 to 70 nm) was demonstrated experimentally for the Roseobacter clade member Silicibacter pomeroyi DSS-3, and intraspecific gene transfer was documented. GTA homologs are surprisingly infrequent in marine metagenomic sequence data, however, and the role of this lateral gene transfer mechanism in ocean bacterioplankton communities remains unclear.
Figures



References
-
- Bergh, O., K. Y. Borsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. - PubMed
-
- Dufresne, A., M. Salanoubat, F. Partensky, F. Artiguenave, I. M. Axmann, V. Barbe, S. Duprat, M. Y. Galperin, E. V. Koonin, F. Le Gall, K. S. Makarova, M. Ostrowski, S. Oztas, C. Robert, I. B. Rogozin, D. J. Scanlan, N. T. de Marsac, J. Weissenbach, P. Wincker, Y. I. Wolf, and W. R. Hess. 2003. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl. Acad. Sci. USA 100:10020-10025. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

