Structure-function studies on the active site of the coelenterazine-dependent luciferase from Renilla
- PMID: 18359861
- PMCID: PMC2271170
- DOI: 10.1110/ps.073355508
Structure-function studies on the active site of the coelenterazine-dependent luciferase from Renilla
Abstract
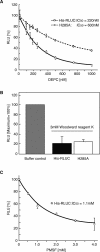
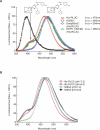
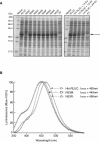
Renilla luciferase (RLUC) is a versatile tool for gene expression assays and in vivo biosensor applications, but its catalytic mechanism remains to be elucidated. RLUC is evolutionarily related to the alpha/beta hydrolase family. Its closest known homologs are bacterial dehalogenases, raising the question of how a protein with a hydrolase fold can function as a decarboxylating oxygenase. Molecular docking simulations with the coelenterazine substrate against an RLUC homology model as well as a recently determined RLUC crystal structure were used to build hypotheses to identify functionally important residues, which were subsequently tested by site-directed mutagenesis, heterologous expression, and bioluminescence emission spectroscopy. The data highlighted two triads of residues that are critical for catalysis. The putative catalytic triad residues D120, E144, and H285 bear only limited resemblance to those found in the active site of aequorin, a coelenterazine-utilizing photoprotein, suggesting that the reaction scheme employed by RLUC differs substantially from the one established for aequorin. The role of H285 in catalysis was further supported by inhibition using diethylpyrocarbonate. Multiple substitutions of N53, W121, and P220--three other residues implicated in product binding in the homologous dehalogenase Sphingomonas LinB--also supported their involvement in catalysis. Together with luminescence spectra, our data lead us to propose that the conserved catalytic triad of RLUC is directly involved in the decarboxylation reaction of coelenterazine to produce bioluminescence, while the other active-site residues are used for binding of the substrate.
Figures


References
-
- Deng, L., Markova, S.V., Vysotski, E.S., Liu, Z.J., Lee, J., Rose, J., Wang, B.C. Crystal structure of a Ca2+-discharged photoprotein: Implications for mechanisms of the calcium trigger and bioluminescence. J. Biol. Chem. 2004;279:33647–33652. - PubMed
-
- Frerichs-Deeken, U., Ranguelova, K., Kappl, R., Huttermann, J., Fetzner, S. Dioxygenases without requirement for cofactors and their chemical model reaction: Compulsory order ternary complex mechanism of 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase involving general base catalysis by histidine 251 and single-electron oxidation of the substrate dianion. Biochemistry. 2004;43:14485–14499. - PubMed
-
- Hart, R.C., Matthews, J.C., Hori, K., Cormier, M.J. Renilla reniformis bioluminescence: Luciferase-catalyzed production of non-radiating excited states from luciferin analogues and elucidation of the excited state species involved in energy transfer to Renilla green fluorescent protein. Biochemistry. 1979;18:2204–2210. - PubMed
-
- Head, J.F., Inouye, S., Teranishi, K., Shimomura, O. The crystal structure of the photoprotein aequorin at 2.3 Å resolution. Nature. 2000;405:372–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

