Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium
- PMID: 18359883
- PMCID: PMC2749499
- DOI: 10.1152/ajplung.00526.2007
Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium
Abstract
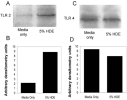
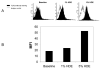
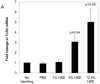
Hog confinement workers are at high risk to develop chronic bronchitis as a result of their exposure to organic dust. Chronic bronchitis is characterized by inflammatory changes of the airway epithelium. A key mediator in inflammation is Toll-like receptor 2 (TLR2). We investigated the role of TLR2 in pulmonary inflammation induced by hog confinement dust. Normal human bronchial epithelial cells (NHBE) were grown in culture and exposed to hog confinement dust extract. Hog confinement dust upregulated airway epithelial cell TLR2 mRNA in a concentration- and time-dependent manner using real-time PCR. There was a similar increase in TLR2 protein at 48 h as shown by Western blot. TLR2 was upregulated on the surface of airway epithelial cells as shown by flow cytometry. A similar upregulation of pulmonary TLR2 mRNA and protein was shown in a murine model of hog confinement dust exposure. Hog confinement dust is known to stimulate epithelial cells to produce IL-6. To determine whether TLR2 expression was being regulated by IL-6, the production of IL-6 was blocked using an IL-6-neutralizing antibody. This resulted in attenuation of the dust-induced upregulation of TLR2. To further demonstrate the importance of IL-6 in the regulation of TLR2, NHBE were directly stimulated with recombinant human IL-6. IL-6 alone was able to upregulate TLR2 in airway epithelial cells. Hog confinement dust upregulates TLR2 in the airway epithelium through an IL-6-dependent mechanism.
Figures





References
-
- Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–892. - PubMed
-
- Becker S, Dailey L, Soukup JM, Silbajoris R, Devlin RB. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol Appl Pharmacol. 2005;203:45–52. - PubMed
-
- Boasen J, Chisholm D, Lebet L, Akira S, Horner AA. House dust extracts elicit Toll-like receptor-dependent dendritic cell responses. J Allergy Clin Immunol. 2005;116:185–191. - PubMed
-
- Donham KJ, Scallon LJ, Popendorf W, Treuhaft MW, Roberts RC. Characterization of dusts collected from swine confinement buildings. Am Ind Hyg Assoc J. 1986;47:404–410. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

