Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity?
- PMID: 18360283
- PMCID: PMC12382384
- DOI: 10.1097/PAS.0b013e318159371c
Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity?
Abstract
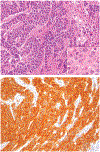
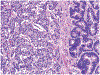
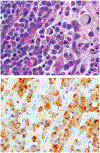
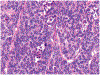
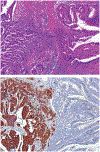
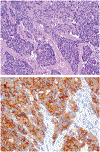
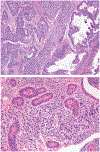
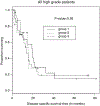
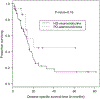
Although small cell carcinoma of the gastrointestinal (GI) tract is well-recognized, nonsmall cell type high-grade neuroendocrine carcinoma (HGNEC) of this site remains undefined. At the current time, neither the World Health Organization nor American Joint Committee on Cancer includes this condition in the histologic classifications, and consequently it is being diagnosed and treated inconsistently. In this study, we aimed at delineating the histologic and immunophenotypical spectrum of HGNECs of the GI tract with emphasis on histologic subtypes. Guided primarily by the World Health Organization/International Association for the Study of Lung Cancer criteria for pulmonary neuroendocrine tumors, we were able to classify 87 high-grade GI tract tumors that initially carried a diagnosis of either poorly differentiated carcinoma with or without any neuroendocrine characteristics, small cell carcinoma, or combined adenocarcinoma-neuroendocrine carcinoma into the following 4 categories. The first was small cell carcinoma (n=23), which had features typical of pulmonary small cell carcinoma, although the cells tended to have a more round nuclear contour. The second was large cell neuroendocrine carcinoma (n=31), which had a morphology similar to its pulmonary counterpart and showed positive immunoreactivity for either chromogranin (71%) or synaptophysin (94%) or both. The third was mixed neuroendocrine carcinoma (n=11), which had intermediate histologic features (eg, cells with an increased nuclear/cytoplasmic ratio but with apparent nucleoli), and positive immunoreactivity for at least 1 neuroendocrine marker. The fourth was poorly differentiated adenocarcinoma (n=17). In addition, 5 of the 87 tumors showed either nonsmall cell type neuroendocrine morphology (n=3) or immunohistochemical reactivity for neuroendocrine markers (n=2), but not both. Further analysis showed that most HGNECs arising in the squamous lined parts (esophagus and anal canal) were small cell type (78%), whereas most involving the glandular mucosa were large cell (53%) or mixed (82%) type; associated adenocarcinomas were more frequent in large cell (61%) or mixed (36%) type than in small cell type (26%); and focal intracytoplasmic mucin was seen only in large cell or mixed type. As a group, the 2-year disease-specific survival for patients with HGNEC was 25.4% (median follow-up time, 11.3 mo). No significant survival difference was observed among the different histologic subtypes. In conclusion, our study demonstrates the existence of both small cell and nonsmall cell types of HGNEC in the GI tract, and provides a detailed illustration of their morphologic spectrum. There are differences in certain pathologic features between small cell and nonsmall cell types, whereas the differences between the subtypes of nonsmall cell category (large cell versus mixed) are less distinct. Given the current uncertainty as to whether large cell neuroendocrine carcinoma is as chemosensitive as small cell carcinoma even in the lung, our data provide further evidence in favor of a dichotomous classification scheme (small cell vs. nonsmall cell) for HGNEC of the GI tract. Separation of nonsmall cell type into large cell and mixed subtypes may not be necessary. These tumors are clinically aggressive. Prospective studies using defined diagnostic criteria are needed to determine their biologic characteristics and optimal management.
Figures




References
-
- Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000.
-
- Bernick PE, Klimstra DS, Shia J, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163–169. - PubMed
-
- Brenner B, Tang LH, Klimstra DS, et al. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730–2739. - PubMed
-
- Burke AB, Shekitka KM, Sobin LH. Small cell carcinomas of the large intestine. Am J Clin Pathol. 1991;95:315–321. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

