Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control
- PMID: 18360571
- PMCID: PMC1661636
Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control
Abstract
Opioid receptors are widely expressed in the central and peripheral nervous system as well as in numerous nonneuronal tissues. Both animal models and human clinical data support the involvement of peripheral opioid receptors in analgesia, particularly in inflammation where both opioid receptor expression and efficacy are increased. Immune cells have been shown to contain numerous opioid peptides such as beta-endorphin (END), met-enkephalin (ENK), and dynorphin-A (DYN), although the predominant opioid peptide involved in immune-cell mediated antinociception is thought to be END. These opioid-containing immune cells migrate to inflamed tissues during a complex process of recruitment by chemokines, adhesion, and extravasation. In these tissues, opioid peptide is released from the immune cells upon stimulation with corticotrophin-releasing factor (CRF), noradrenaline, and interleukin 1beta (IL-1beta), and the immune cells return to the local lymph node depleted of peptide. Consistent with this model, systemic immunosuppression may lead to impaired endogenous analgesia as competent immune cells are essential to achieve release of endogenous opioid peptides within inflamed tissue. A further level of complexity is added by the observation that exogenous opioids may impair immune cell function, although there is some evidence to suggest that endogenous opioid peptides do not share this immunosuppressive effect. Improving our understanding of endogenous opioid mechanisms will provide valuable insight towards the development of novel treatments for pain with improved side effect profiles.
Figures
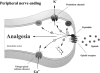

References
-
- Abbadie C, Pan Y, Pasternak G. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000;419:244–56. - PubMed
-
- Akil H, Meng F, Mansour A, et al. Cloning and characterization of multiple opioid receptors. NIDA Res Monogr. 1996;161:127–40. - PubMed
-
- Akil H, Young E, Watson SJ, et al. Opiate binding properties of naturally occurring N- and C-terminus modified beta-endorphins. Peptides. 1981;2:289–92. - PubMed
-
- Alicea C, Belkowski S, Eisenstein TK, et al. Inhibition of primary murine macrophage cytokine production in vitro following treatment with the kappa-opioid agonist U50,488H. J Neuroimmunol. 1996;64:83–90. - PubMed
-
- Andersson SE, Lexmuller K, Johansson A, et al. Tissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the rat. J Rheumatol. 1999;26:2018–24. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
