Ibandronate: the first once-monthly oral bisphosphonate for treatment of postmenopausal osteoporosis
- PMID: 18360577
- PMCID: PMC1661644
Ibandronate: the first once-monthly oral bisphosphonate for treatment of postmenopausal osteoporosis
Abstract
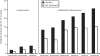
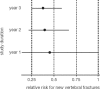
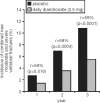
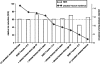
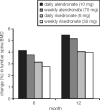
Osteoporosis is a major healthcare problem that continues growing as the population ages. Sufferers become increasingly susceptible to fractures, which compromise physical and emotional health and increase healthcare costs. Bisphosphonates are the most widely used medicines for the treatment and prevention of postmenopausal osteoporosis. However, therapeutic adherence is suboptimal, meaning that outcomes demonstrated in clinical trials are not realized in the real world. It is anticipated that reducing dosing frequency may facilitate medication intake and thereby improve adherence. Ibandronate is a potent nitrogen-containing bisphosphonate specifically developed for administration with long dose-free intervals. The comprehensive ibandronate preclinical development program has demonstrated dose-dependent improvements or preservation of bone quality and strength. The feasibility of intermittent dosing using the same total dose level as continuous dosing was also confirmed. In postmenopausal osteoporosis, once-monthly oral ibandronate has been shown to be therapeutically equivalent and even superior to daily oral ibandronate, which has demonstrated antifracture efficacy for vertebral and nonvertebral fractures, bone mineral density gains at the spine and hip, and reduction in bone resorption to premenopausal levels. Once-monthly oral ibandronate is also associated with excellent safety and tolerability, and promises to further improve therapeutic adherence to bisphosphonate treatment, thereby enhancing therapeutic outcomes.
Figures
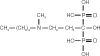
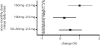
References
-
- Barrett J, Worth E, Bauss F, et al. Ibandronate: A clinical pharmacological and pharmacokinetic update. J Clin Pharmacol. 2004;44:951–65. - PubMed
-
- Bartl R, Goette S, Hadji P, et al. Persistence and compliance with daily- and weekly-administered bisphosphonates for osteoporosis in Germany [abstract p195] Osteoporos Int. 2005;16(Suppl 3):S45.
-
- Bauss F, Lalla S, Endele R, et al. The effects of treatment with ibandronate on bone mass, architecture, biomechanical properties and bone concentration of ibandronate in ovariectomized aged rats. J Rheumatol. 2002;29:2200–8. - PubMed
-
- Bauss F, Russell RGG. Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing. Osteoporos Int. 2004;15:423–33. - PubMed
LinkOut - more resources
Full Text Sources
