The use of amisulpride in the treatment of acute psychosis
- PMID: 18360610
- PMCID: PMC1936283
- DOI: 10.2147/tcrm.2007.3.1.3
The use of amisulpride in the treatment of acute psychosis
Abstract
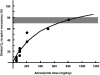
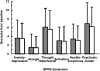
The management of acute episodes in schizophrenia is frequently initiated in the psychiatric emergency department and requires rapid intervention to relieve distress and psychiatric symptoms. Both non-pharmacological and pharmacological interventions are needed to calm the patient and prevent potential harm to the patient or others. Treatment is a step-by-step process including management of behavioral symptomatology, diagnosis of potential organic causes, and evaluation of potential substance abuse. Better care is delivered if predefined standard operating procedures are adopted systematically. The ultimate goal of treatment is to establish a therapeutic alliance with the patient. Atypical antipsychotics given orally are recommended as a first-line treatment. As the treatment endpoint is calmness rather than sleep, a non-sedative antipsychotic agent is usually preferred. Drug tolerance is a major issue for the patient. Amisulpride is an effective atypical antipsychotic agent in this context. The optimal dose is 800 mg/day, which is effective on positive and negative symptoms and can be given from the first day with a low risk of extrapyramidal symptoms. Since drug-drug interactions are limited, agitation and anxiety may be controlled by short-term adjunctive therapy with benzodiazepines. In conclusion, amisulpride is an appropriate first-line treatment for the management of acute psychosis.
Figures


Similar articles
-
Amisulpride: a review of its use in the management of schizophrenia.Drugs. 2001;61(14):2123-50. doi: 10.2165/00003495-200161140-00014. Drugs. 2001. PMID: 11735643 Review.
-
Practical issues with amisulpride in the management of patients with schizophrenia.Clin Drug Investig. 2008;28(8):465-77. doi: 10.2165/00044011-200828080-00001. Clin Drug Investig. 2008. PMID: 18598092 Review.
-
Spotlight on amisulpride in schizophrenia.CNS Drugs. 2002;16(3):207-11. doi: 10.2165/00023210-200216030-00007. CNS Drugs. 2002. PMID: 11888341 Review.
-
Switching to amisulpride.Curr Med Res Opin. 2002;18 Suppl 3:s23-8. doi: 10.1185/030079902125001092. Curr Med Res Opin. 2002. PMID: 12418609 Review.
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
Cited by
-
A 6-week, randomized, multicentre, open-label study comparing efficacy and tolerability of amisulpride at a starting dose of 400 mg/day versus 800 mg/day in patients with acute exacerbations of schizophrenia.Clin Drug Investig. 2012 Nov;32(11):735-45. doi: 10.1007/s40261-012-0002-8. Clin Drug Investig. 2012. PMID: 23018281 Clinical Trial.
-
Management of patients presenting with acute psychotic episodes of schizophrenia.CNS Drugs. 2009;23(3):193-212. doi: 10.2165/00023210-200923030-00002. CNS Drugs. 2009. PMID: 19320529 Review.
-
Discovery of Nonracemic Amisulpride to Maximize Benefit/Risk of 5-HT7 and D2 Receptor Antagonism for the Treatment of Mood Disorders.Clin Pharmacol Ther. 2021 Sep;110(3):808-815. doi: 10.1002/cpt.2282. Epub 2021 Jun 12. Clin Pharmacol Ther. 2021. PMID: 33961287 Free PMC article. Clinical Trial.
-
Prepandemic psychotropic drug status in Portugal: a nationwide pharmacoepidemiological profile.Sci Rep. 2023 Apr 27;13(1):6912. doi: 10.1038/s41598-023-33765-0. Sci Rep. 2023. PMID: 37106018 Free PMC article.
-
Update on the management of symptoms in schizophrenia: focus on amisulpride.Neuropsychiatr Dis Treat. 2009;5:267-77. doi: 10.2147/ndt.s3949. Epub 2009 May 20. Neuropsychiatr Dis Treat. 2009. PMID: 19557121 Free PMC article.
References
-
- Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies The Expert Consensus Guideline Series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1–88. - PubMed
-
- Barbee JG, Mancuso DM, Freed CR, et al. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149:506–10. - PubMed
-
- Battaglia J. Pharmacological management of acute agitation. Drugs. 2005;65:1207–22. - PubMed
-
- Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15:335–40. - PubMed
-
- Canal M, Gilet G, Simpson H. Lack of drug interaction potential of amisulpride P01–503. Int J Neuropsychopharmacology. 2004;7:S265.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

