Structural features responsible for the biological stability of Histoplasma's virulence factor CBP
- PMID: 18361504
- PMCID: PMC3124767
- DOI: 10.1021/bi701495v
Structural features responsible for the biological stability of Histoplasma's virulence factor CBP
Abstract
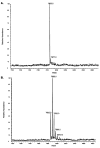
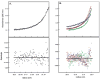
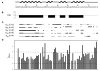
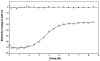
The virulence factor CBP is the most abundant protein secreted by Histoplasma capsulatum, a pathogenic fungus that causes histoplasmosis. Although the biochemical function and pathogenic mechanism of CBP are unknown, quantitative Ca (2+) binding measurements indicate that CBP has a strong affinity for calcium ( K D = 6.45 +/- 0.4 nM). However, no change in structure was observed upon binding of calcium, prompting a more thorough investigation of the molecular properties of CBP with respect to self-association, secondary structure, and stability. Over a wide range of pH values and salt concentrations, CBP exists predominantly as a stable, noncovalent homodimer in both its calcium-free and -bound states. Solution-state NMR and circular dichroism (CD) measurements indicated that the protein is largely alpha-helical, and its secondary structure content changes little over the range of pH values encountered physiologically. ESI-MS revealed that the six cysteine residues of CBP are involved in three intramolecular disulfide bonds that help maintain a highly protease resistant structure. Thermally and chemically induced denaturation studies indicated that unfolding of disulfide-intact CBP is reversible and provided quantitative measurements of protein stability. This disulfide-linked, protease resistant, homodimeric alpha-helical structure of CBP is likely to be advantageous for a virulence factor that must survive the harsh environment within the phagolysosomes of host macrophages.
Figures
References
-
- Eissenberg LG, Goldman WE. Fusion of lysosomes with phagosomes containing Histoplasma capsulatum: Use of fluoresceinated dextran. Adv Exp Med Biol. 1988;239:53–61. - PubMed
-
- Sebghati TS, Engle JT, Goldman WE. Intracellular parasitism by Histoplasma capsulatum: Fungal virulence and calcium dependence. Science. 2000;290:1368–1372. - PubMed
-
- Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for α-(1,3)-glucan in virulence. Mol Microbiol. 2004;53:153–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

