Ionic dysregulatory phenotyping of pathologic retinal thinning with manganese-enhanced MRI
- PMID: 18362105
- PMCID: PMC2517149
- DOI: 10.1167/iovs.08-1720
Ionic dysregulatory phenotyping of pathologic retinal thinning with manganese-enhanced MRI
Abstract
Purpose: To test the hypothesis that manganese-enhanced MRI (MEMRI) provides a sensitive and robust measure of an important retinal ionic dysregulatory phenotype in pathologic retinal thinning.
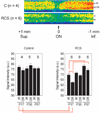
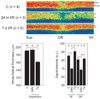
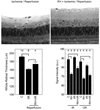
Methods: Four hours after intraperitoneal MnCl(2) injection, high-resolution MEMRI data were collected from overnight dark-adapted male control Sprague-Dawley and albino Royal College of Surgeons rats before (at development stage postnatal day [P] 17) and during photoreceptor degeneration (P36 and P57). In separate experiments, control rats, with and without repetitive hypoxic preconditioning, were subjected to high IOP (100 mm Hg) for 60 minutes followed by 24 hours or 7 days of reperfusion (e.g., ischemia/reperfusion). Central retinal thickness and intraretinal ion activity were measured from the MEMRI data. Histology examination was also performed to confirm retinal damage.
Results: In two different neurodegenerative models, MEMRI revealed first-time evidence for changes (P < 0.05) in intraretinal ion regulation before and during pathologic, but not (P > 0.05) developmental, retinal thinning. This phenotype was significantly altered by a neuroprotective repetitive hypoxic preconditioning protocol.
Conclusions: MEMRI and a nontoxic systemic dose of MnCl(2) provided an objective, noninvasive measure of an ionic deregulatory phenotype that appears useful for improved early diagnosis and treatment prognosis in a range of neurodegenerative diseases and their treatment.
Figures

References
-
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. - PubMed
-
- Ohguro H, Ohguro I, Mamiya K, Maeda T, Nakazawa M. Prolonged survival of the phosphorylated form of rhodopsin during dark adaptation of Royal College Surgeons rat. FEBS Lett. 2003;551:128–132. - PubMed
-
- Kalloniatis M, Tomisich G, Wellard JW, Foster LE. Mapping photoreceptor and postreceptoral labelling patterns using a channel permeable probe (agmatine) during development in the normal and RCS rat retina. Vis Neurosci. 2002;19:61–70. - PubMed
-
- Fox DA, Poblenz AT, He L, Harris JB, Medrano CJ. Pharmacological strategies to block rod photoreceptor apoptosis caused by calcium overload: a mechanistic target-site approach to neuroprotection. Eur J Ophthalmol. 2003;13 suppl 3:S44–S56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

