Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines
- PMID: 18362128
- PMCID: PMC2423104
- DOI: 10.1128/IAI.00033-08
Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines
Abstract
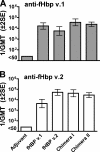
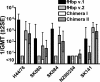
Factor H-binding protein (fHbp) is a novel meningococcal vaccine candidate that elicits serum antibodies that activate classical complement pathway bacteriolysis and also inhibit binding of the complement down-regulatory protein, factor H, to the bacterial surface. One limitation of fHbp as a vaccine candidate is antigenic variability, since antibodies to fHbp in the variant 1 (v.1) antigenic group do not protect against strains expressing v.2 or v.3 proteins, and vice versa. We have identified amino acid residues of epitopes recognized by bactericidal anti-fHbp monoclonal antibodies prepared against fHbp from each of the variant groups. One epitope expressed by nearly all v.1 proteins mapped to the B domain, while epitopes expressed by fHbp v.2 or v.3 mapped to the C domain. The results provided the rationale for engineering chimeric fHbp molecules containing the A domain (which is conserved across all variant groups), a portion of the B domain of a v.1 protein, and the carboxyl-terminal portion of the B domain and the C domain of a v.2 protein. By enzyme-linked immunosorbent assay, the resulting recombinant chimeric proteins expressed epitopes from all three variant groups. In mice, the chimeric vaccines elicited serum antibodies with bactericidal activity against a panel of genetically diverse strains expressing fHbp v.1, v.2, or v.3. The data demonstrate the feasibility of preparing a meningococcal vaccine from a single recombinant protein that elicits broad bactericidal activity, including group B strains, which account for 50 percent of cases of meningococcal disease and for which there currently is no broadly protective vaccine.
Figures

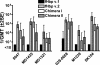
References
-
- Amadou Hamidou, A., S. Djibo, A. Elhaj Mahamane, A. Moussa, H. Findlow, F. Sidikou, R. Cisse, A. Garba, R. Borrow, S. Chanteau, and P. Boisier. 2006. Prospective survey on carriage of Neisseria meningitidis and protective immunity to meningococci in schoolchildren in Niamey (Niger): focus on serogroup W135. Microbes Infect. 82098-2104. - PubMed
-
- Baker, M. G., D. R. Martin, C. E. Kieft, and D. Lennon. 2001. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991-2000. J. Paediatr. Child Health 37S13-S19. - PubMed
-
- Beernink, P. T., J. A. Welsch, L. H. Harrison, A. Leipus, S. L. Kaplan, and D. M. Granoff. 2007. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J. Infect. Dis. 1951472-1479. - PMC - PubMed
-
- Berild, D., T. W. Gedde-Dahl, and T. Abrahamsen. 1980. Meningococcal disease in the Norwegian Armed Forces 1967-1979. Some epidemiological aspects. NIPH Ann. 323-30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

