Evidence that proline focuses movement of the floppy loop of arylalkylamine N-acetyltransferase (EC 2.3.1.87)
- PMID: 18362150
- PMCID: PMC2386931
- DOI: 10.1074/jbc.M800593200
Evidence that proline focuses movement of the floppy loop of arylalkylamine N-acetyltransferase (EC 2.3.1.87)
Abstract
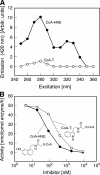
Arylalkylamine N-acetyltransferase (AANAT) catalyzes the N-acetylation of serotonin, the penultimate step in the synthesis of melatonin. Pineal AANAT activity increases at night in all vertebrates, resulting in increased melatonin production. This increases circulating levels of melatonin, thereby providing a hormonal signal of darkness. Kinetic and structural analysis of AANAT has determined that one element is floppy. This element, termed Loop 1, is one of three loops that comprise the arylalkylamine binding pocket. During the course of chordate evolution, Loop 1 acquired the tripeptide CPL, and the enzyme became highly active. Here we focused on the functional importance of the CPL tripeptide and found that activity was markedly reduced when it was absent. Moreover, increasing the local flexibility of this tripeptide region by P64G and P64A mutations had the counterintuitive effect of reducing activity and reducing the overall movement of Loop 1, as estimated from Langevin dynamics simulations. Binding studies indicate that these mutations increased the off-rate constant of a model substrate without altering the dissociation constant. The structural kink and local rigidity imposed by Pro-64 may enhance activity by favoring configurations of Loop 1 that facilitate catalysis and do not become immobilized by intramolecular interactions.
Figures






References
-
- Neuwald, A. F., and Landsman, D. (1997) Trends Biochem. Sci. 22 154-155 - PubMed
-
- Vetting, M. W. S., de Carvalho, L., Yu, M., Hegde, S. S., Magnet, S., Roderick, S. L., and Blanchard, J. S. (2005) Arch. Biochem. Biophys. 433 212-226 - PubMed
-
- Coon, S. L., Roseboom, P., Baler, R., Weller, J. L., Namboodiri, M. A., Koonin, E. V., and Klein, D. C. (1995) Science 270 1681-1683 - PubMed
-
- Klein, D. C., Coon, S. L., Roseboom, P. H., Weller, J. L., Bernard, M., Gastel, J. A., Zatz, M., Iuvone, P. M., Rodriguez, I. R., Begay, V., Falcon, J., Cahill, G. M., Cassone, V. M., and Baler, R. (1997) Recent Prog. Horm. Res. 52 307-357 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
