Regulatory and structural differences in the Cu,Zn-superoxide dismutases of Salmonella enterica and their significance for virulence
- PMID: 18362154
- PMCID: PMC2376220
- DOI: 10.1074/jbc.M710499200
Regulatory and structural differences in the Cu,Zn-superoxide dismutases of Salmonella enterica and their significance for virulence
Abstract
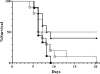
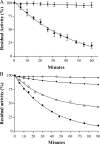
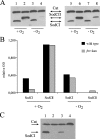
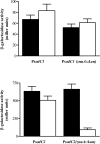
Many of the most virulent strains of Salmonella enterica produce two distinct Cu,Zn-superoxide dismutases (SodCI and SodCII). The bacteriophage-encoded SodCI enzyme makes the greater contribution to Salmonella virulence. We have performed a detailed comparison of the functional, structural, and regulatory properties of the Salmonella SodC enzymes. Here we demonstrate that SodCI and SodCII differ with regard to specific activity, protease resistance, metal affinity, and peroxidative activity, with dimeric SodCI exhibiting superior stability and activity. In particular, monomeric SodCII is unable to retain its catalytic copper ion in the absence of zinc. We have also found that SodCI and SodCII are differentially affected by oxygen, zinc availability, and the transcriptional regulator FNR. SodCII is strongly down-regulated under anaerobic conditions and dependent on the high affinity ZnuABC zinc transport system, whereas SodCI accumulation in vitro and within macrophages is FNR-dependent. We have confirmed earlier findings that SodCII accumulation in intracellular Salmonella is negligible, whereas SodCI is strongly up-regulated in macrophages. Our observations demonstrate that differences in expression, activity, and stability help to account for the unique contribution of the bacteriophage-encoded SodCI enzyme to Salmonella virulence.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

