Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways
- PMID: 18362162
- PMCID: PMC2423292
- DOI: 10.1128/MCB.00040-08
Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways
Abstract
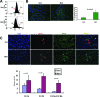
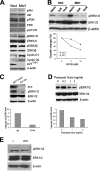
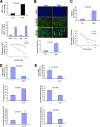
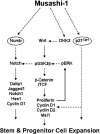
The RNA-binding protein Musashi1 (Msi1) is a positive regulator of Notch-mediated transcription in Drosophila melanogaster and neural progenitor cells and has been identified as a putative human breast stem cell marker. Here we describe a novel functional role for Msi1: its ability to drive progenitor cell expansion along the luminal and myoepithelial lineages. Expression of Msi1 in mammary epithelial cells increases the abundance of CD24(hi) Sca-1(+), CD24(hi) CD29(+), CK19, CK6, and double-positive CK14/CK18 progenitor cells. Proliferation is associated with increased proliferin-1 (PLF1) and reduced Dickkopf-3 (DKK3) secretion into the conditioned medium from Msi-expressing cells, which is associated with increased colony formation and extracellular signal-regulated kinase (ERK) phosphorylation. Treatment with the MEK inhibitor U0126 inhibits ERK activation and decreases Notch and beta-catenin/T-cell factor (TCF) reporter activity resulting from Msi1 expression. Reduction of DKK3 in control cells with a short hairpin RNA (shRNA) increases Notch and beta-catenin/TCF activation, whereas reduction of PLF1 with a shRNA in Msi1-expressing cells inhibits these pathways. These results identify Msi1 as a key determinant of the mammary lineage through its ability to coordinate cell cycle entry and activate the Notch and Wnt pathways by a novel autocrine process involving PLF1 and DKK3.
Figures

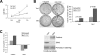
References
-
- Abarzua, F., M. Sakaguchi, M. Takaishi, Y. Nasu, K. Kurose, S. Ebara, M. Miyazaki, M. Namba, H. Kumon, and N. H. Huh. 2005. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 659617-9622. - PubMed
-
- Baron, M. 2003. An overview of the Notch signalling pathway. Semin. Cell Dev. Biol. 14113-119. - PubMed
-
- Battelli, C., G. N. Nikopoulos, J. G. Mitchell, and J. M. Verdi. 2006. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21WAF-1. Mol. Cell. Neurosci. 3185-96. - PubMed
-
- Baumann, P., N. Cremers, F. Kroese, G. Orend, R. Chiquet-Ehrismann, T. Uede, H. Yagita, and J. P. Sleeman. 2005. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 6510783-10793. - PubMed
-
- Bocchinfuso, W. P., W. P. Hively, J. F. Couse, H. E. Varmus, and K. S. Korach. 1999. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 591869-1876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
