p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity
- PMID: 18362168
- PMCID: PMC2423160
- DOI: 10.1128/MCB.02246-07
p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity
Abstract
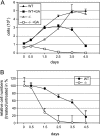
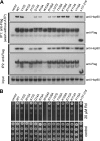
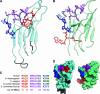
The molecular chaperone Hsp90 assists a subset of cellular proteins and is essential in eukaryotes. A cohort of cochaperones contributes to and regulates the multicomponent Hsp90 machine. Unlike the biochemical activities of the cochaperone p23, its in vivo functions and the structure-function relationship remain poorly understood, even in the genetically tractable model organism Saccharomyces cerevisiae. The SBA1 gene that encodes the p23 ortholog in this species is not an essential gene. We found that in the absence of p23/Sba1p, yeast and mammalian cells are hypersensitive to Hsp90 inhibitors. This protective function of Sba1p depends on its abilities to bind Hsp90 and to block the Hsp90 ATPase and inhibitor binding. In contrast, the protective function of Sba1p does not require the Hsp90-independent molecular chaperone activity of Sba1p. The structure-function analysis suggests that Sba1p undergoes considerable structural rearrangements upon binding Hsp90 and that the large size of the p23/Sba1p-Hsp90 interaction surface facilitates maintenance of high affinity despite sequence divergence during evolution. The large interface may also contribute to preserving a protective function in an environment in which Hsp90 inhibitory compounds can be produced by various microorganisms.
Figures






Similar articles
-
Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle.Nat Commun. 2021 Feb 5;12(1):828. doi: 10.1038/s41467-021-21063-0. Nat Commun. 2021. PMID: 33547294 Free PMC article.
-
SBA1 encodes a yeast hsp90 cochaperone that is homologous to vertebrate p23 proteins.Mol Cell Biol. 1998 Jul;18(7):3727-34. doi: 10.1128/MCB.18.7.3727. Mol Cell Biol. 1998. PMID: 9632755 Free PMC article.
-
The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies.Genes Dev. 2000 Feb 15;14(4):422-34. Genes Dev. 2000. PMID: 10691735 Free PMC article.
-
p23 and Aha1.Subcell Biochem. 2015;78:113-31. doi: 10.1007/978-3-319-11731-7_6. Subcell Biochem. 2015. PMID: 25487019 Review.
-
p23, a simple protein with complex activities.Cell Stress Chaperones. 2003 Summer;8(2):108-13. doi: 10.1379/1466-1268(2003)008<0108:paspwc>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627195 Free PMC article. Review.
Cited by
-
Hypoviral-regulated HSP90 co-chaperone p23 (CpCop23) determines the colony morphology, virulence, and viral response of chestnut blight fungus Cryphonectria parasitica.Mol Plant Pathol. 2023 May;24(5):413-424. doi: 10.1111/mpp.13308. Epub 2023 Feb 10. Mol Plant Pathol. 2023. PMID: 36762926 Free PMC article.
-
Hsp90 inhibitors radicicol and geldanamycin have opposing effects on Leishmania Aha1-dependent proliferation.Cell Stress Chaperones. 2017 Sep;22(5):729-742. doi: 10.1007/s12192-017-0800-2. Epub 2017 Apr 28. Cell Stress Chaperones. 2017. PMID: 28455612 Free PMC article.
-
Hsp90 inhibition: elimination of shock and stress.Bioorg Med Chem Lett. 2010 Sep 1;20(17):4983-7. doi: 10.1016/j.bmcl.2010.06.108. Epub 2010 Jul 1. Bioorg Med Chem Lett. 2010. PMID: 20656483 Free PMC article.
-
Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies.Future Med Chem. 2011 Sep;3(12):1523-37. doi: 10.4155/fmc.11.88. Future Med Chem. 2011. PMID: 21882945 Free PMC article. Review.
-
Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity.Future Med Chem. 2013 Jun;5(9):1059-71. doi: 10.4155/fmc.13.88. Future Med Chem. 2013. PMID: 23734688 Free PMC article. Review.
References
-
- Bandhakavi, S., R. O. McCann, D. E. Hanna, and C. V. Glover. 2003. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J. Biol. Chem. 2782829-2836. - PubMed
-
- Bose, S., T. Weikl, H. Bügl, and J. Buchner. 1996. Chaperone function of Hsp90-associated proteins. Science 2741715-1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
