Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb
- PMID: 18362174
- PMCID: PMC2292226
- DOI: 10.1084/jem.20072565
Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb
Abstract
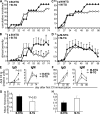
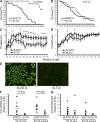
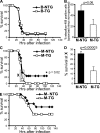
FcgammaRIIb is an inhibitory Fc receptor expressed on B cells and myeloid cells. It is important in controlling responses to infection, and reduced expression or function predisposes to autoimmunity. To determine if increased expression of FcgammaRIIb can modulate these processes, we created transgenic mice overexpressing FcgammaRIIb on B cells or macrophages. Overexpression of FcgammaRIIb on B cells reduced the immunoglobulin G component of T-dependent immune responses, led to early resolution of collagen-induced arthritis (CIA), and reduced spontaneous systemic lupus erythematosus (SLE). In contrast, overexpression on macrophages had no effect on immune responses, CIA, or SLE but increased mortality after Streptococcus pneumoniae infection. These results help define the role of FcgammaRIIb in immune responses, demonstrate the contrasting roles played by FcgammaRIIb on B cells and macrophages in the control of infection and autoimmunity, and emphasize the therapeutic potential for modulation of FcgammaRIIb expression on B cells in inflammatory and autoimmune disease.
Figures




References
-
- Nimmerjahn, F., and J.V. Ravetch. 2006. Fcgamma receptors: old friends and new family members. Immunity. 24:19–28. - PubMed
-
- Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. - PubMed
-
- Xiang, Z., A.J. Cutler, R.J. Brownlie, K. Fairfax, K.E. Lawlor, E. Severinson, E.U. Walker, R.A. Manz, D.M. Tarlinton, and K.G.C. Smith. 2007. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 8:419–429. - PubMed
-
- Takai, T., M. Ono, M. Hikida, H. Ohmori, and J.V. Ravetch. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 379:346–349. - PubMed
-
- Takai, T. 2005. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 25:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

