Antigen kinetics determines immune reactivity
- PMID: 18362362
- PMCID: PMC2278203
- DOI: 10.1073/pnas.0706296105
Antigen kinetics determines immune reactivity
Abstract
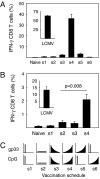
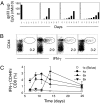
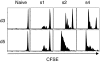
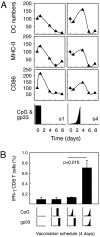
A current paradigm in immunology is that the strength of T cell responses is governed by antigen dose, localization, and costimulatory signals. This study investigates the influence of antigen kinetics on CD8 T cell responses in mice. A fixed cumulative antigen dose was administered by different schedules to produce distinct dose-kinetics. Antigenic stimulation increasing exponentially over days was a stronger stimulus for CD8 T cells and antiviral immunity than a single dose or multiple dosing with daily equal doses. The same was observed for dendritic cell vaccination, with regard to T cell and anti-tumor responses, and for T cells stimulated in vitro. In conclusion, stimulation kinetics per se was shown to be a separate parameter of immunogenicity. These findings warrant a revision of current immunization models and have implications for vaccine development and immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Janeway CA., Jr Cold Spring Harb Symp Quant Biol. 1989;54:1–13. - PubMed
-
- Zinkernagel RM. Science. 1996;271:173–178. - PubMed
-
- Germain RN. Nat Med. 2004;10:1307–1320. - PubMed
-
- Johansen P, Senti G, Martinez Gomez JM, Storni T, von Beust BR, Wuthrich B, Bot A, Kundig TM. Clin Exp Allergy. 2005;35:1591–1598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

