Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma
- PMID: 18362366
- PMCID: PMC3904367
- DOI: 10.1200/JCO.2007.14.1853
Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma
Abstract
Purpose: The long-term impact of thalidomide plus dexamethasone (thal/dex) as primary therapy for newly diagnosed multiple myeloma (MM) is unknown. The goal of this study was to compare thalidomide plus dexamethasone versus placebo plus dexamethasone (placebo/dex)as primary therapy for newly diagnosed MM.
Patients and methods: In this double-blind, placebo-controlled trial, patients with untreated symptomatic MM were randomized to thal/dex (arm A) or to placebo plus dexamethasone (dex) (arm B). Patients in arm A received oral thalidomide 50 mg daily, escalated to 100 mg on day 15, and to 200 mg from day 1 of cycle 2 (28-day cycles). Oral dex 40 mg was administered on days 1 through 4, 9 through 12, and 17 through 20 during cycles 1 through 4 and on days 1 through 4 only from cycle 5 onwards. Patients in arm B received placebo and dex, administered as in arm A. The primary end point of the study was time to progression. This study is registered at http://ClinicalTrials.gov (NCT00057564).
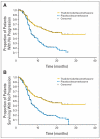
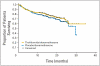
Results: A total of 470 patients were enrolled (235 randomly assigned to thal/dex and 235 to placebo/dex). The overall response rate was significantly higher with thal/dex compared with placebo/dex (63% v 46%), P < .001. Time to progression (TTP) was significantly longer with thal/dex compared with placebo/dex (median, 22.6 v 6.5 months, P < .001). Grade 4 adverse events were more frequent with thal/dex than with placebo/dex (30.3% v 22.8%).
Conclusion: Thal/dex results in significantly higher response rates and significantly prolongs TTP compared with dexamethasone alone in patients with newly diagnosed MM.
Figures
References
-
- Sirohi B, Powles R. Multiple myeloma. Lancet. 2004;363:875–887. - PubMed
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. - PubMed
-
- Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20:4319–4323. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

