Nuclear apoptosis contributes to sarcopenia
- PMID: 18362685
- PMCID: PMC2778230
- DOI: 10.1097/JES.0b013e318168e9dc
Nuclear apoptosis contributes to sarcopenia
Abstract
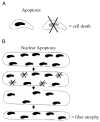
Apoptosis results in DNA fragmentation and, subsequently, destruction of cells containing a single nucleus. Our hypothesis is that multinucleated cells such as muscle fibers can experience apoptotic-induced loss of single nuclei (nuclear apoptosis) without destruction of the entire fiber. The loss of nuclei likely contributes to atrophy and sarcopenia. Furthermore, increased chronic activity attenuates apoptotic signaling, which may reduce sarcopenia.
Figures


References
-
- Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. - PubMed
-
- Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am. J. Physiol. Cell Physiol. 2002;283:C66–C76. - PubMed
-
- Alway SE, Siu PM, Murlasits Z, Butler DC. Muscle hypertrophy models: applications for research on aging. Can. J. Appl. Physiol. 2005;30:591–624. - PubMed
-
- Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell Sci. 2005;118(pt 20):4813–4821. - PubMed
-
- Bruusgaard JC, Liestol K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 2006;100:2024–2030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

