The role of S-1 in the treatment of gastric cancer
- PMID: 18362933
- PMCID: PMC2361710
- DOI: 10.1038/sj.bjc.6604332
The role of S-1 in the treatment of gastric cancer
Abstract
S-1 is a potent antitumour drug in gastric cancer. After years of disagreement about the utility of chemotherapy for advanced gastric cancer, several studies have recently demonstrated the efficacy of S-1 in both the adjuvant and primary settings. In this Minireview, the value of S-1 in the treatment of gastric cancer is discussed.
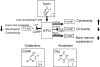
Figures
References
-
- Boku N, Yamamoto S, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Kimura A, Ohtsu A, Gastrointestinal Oncology Study Group/Japan Clinical Oncology Group (2007) Randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG9912). J Clin Oncol 25(18S): 965s (abstract LBA4513)
-
- Chu QS, Hammond LA, Schwartz G, Ochoa L, Rha SY, Denis L, Molpus K, Roedig B, Letrent SP, Damle B, DeCillis AP, Rowinsky EK (2004) Phase I and pharmacokinetic study of the oral fluoropyrimidine S-1 on a once-daily-for-28-day schedule in patients with advanced malignancies. Clin Cancer Res 10(15): 4913–4921 - PubMed
-
- Japanese Gastric Cancer Association (2004) Guideline for Treatment of Gastric Cancer. Kanehara Shuppan: Tokyo
-
- Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki Km, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1999) Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5(8): 2000–2005 - PubMed
-
- Imamura H, Iishi H, Tsuburaya A, Hatake K, Imamoto H, Esaki T, Kato M, Furukawa H, Hamada C, Sakata Y (2008) Irinotecan plus S-l (IRI-S) versus S-l alone as first line treatment for advanced gastric cancer: results of a randomized phase III study (GC0301/TOP-002). ASCO-GI 2008: (abstract 5) www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmview=abst detail_view&conflD=53&abstractID=10303
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

