Circulating plasma factors induce tubular and glomerular alterations in septic burns patients
- PMID: 18364044
- PMCID: PMC2447585
- DOI: 10.1186/cc6848
Circulating plasma factors induce tubular and glomerular alterations in septic burns patients
Abstract
Background: Severe burn is a systemic illness often complicated by sepsis. Kidney is one of the organs invariably affected, and proteinuria is a constant clinical finding. We studied the relationships between proteinuria and patient outcome, severity of renal dysfunction and systemic inflammatory state in burns patients who developed sepsis-associated acute renal failure (ARF). We then tested the hypothesis that plasma in these patients induces apoptosis and functional alterations that could account for proteinuria and severity of renal dysfunction in tubular cells and podocytes.
Methods: We studied the correlation between proteinuria and indexes of systemic inflammation or renal function prospectively in 19 severe burns patients with septic shock and ARF, and we evaluated the effect of plasma on apoptosis, polarity and functional alterations in cultured human tubular cells and podocytes. As controls, we collected plasma from 10 burns patients with septic shock but without ARF, 10 burns patients with septic shock and ARF, 10 non-burns patients with septic shock without ARF, 10 chronic uremic patients and 10 healthy volunteers.
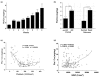
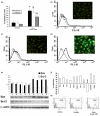
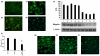
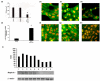
Results: Septic burns patients with ARF presented a severe proteinuria that correlated to outcome, glomerular (creatinine/urea clearance) and tubular (fractional excretion of sodium and potassium) functional impairment and systemic inflammation (white blood cell (WBC) and platelet counts). Plasma from these patients induced a pro-apoptotic effect in tubular cells and podocytes that correlated with the extent of proteinuria. Plasma-induced apoptosis was significantly higher in septic severe burns patients with ARF with respect to those without ARF or with septic shock without burns. Moreover, plasma from septic burns patients induced an alteration of polarity in tubular cells, as well as reduced expression of the tight junction protein ZO-1 and of the endocytic receptor megalin. In podocytes, plasma from septic burns patients increased permeability to albumin and decreased the expression of the slit diaphragm protein nephrin.
Conclusion: Plasma from burns patients with sepsis-associated ARF contains factors that affect the function and survival of tubular cells and podocytes. These factors are likely to be involved in the pathogenesis of acute tubular injury and proteinuria, which is a negative prognostic factor and an index of renal involvement in the systemic inflammatory reaction.
Figures

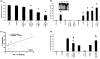
Comment in
-
Circulating pro-apoptotic mediators in burn septic acute renal failure.Crit Care. 2008;12(2):126. doi: 10.1186/cc6798. Epub 2008 Mar 31. Crit Care. 2008. PMID: 18394180 Free PMC article.
References
-
- Camussi G, Mariano F, Biancone L, De Martino A, Bussolati B, Montrucchio G, Tobias PS. Lipopolysaccharide binding protein and CD14 modulate the synthesis of platelet-activating factor by human monocytes and mesangial and endothelial cells stimulated with lipopolysaccharide. J Immunol. 1995;155:316–324. - PubMed
-
- Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol. 2002;168:5817–5823. - PubMed

