Peroxisomes as novel players in cell calcium homeostasis
- PMID: 18364350
- PMCID: PMC3258891
- DOI: 10.1074/jbc.M800648200
Peroxisomes as novel players in cell calcium homeostasis
Abstract
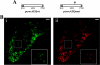
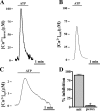
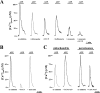
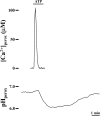
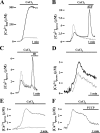
Ca2+ concentration in peroxisomal matrix ([Ca2+](perox)) has been monitored dynamically in mammalian cells expressing variants of Ca2+-sensitive aequorin specifically targeted to peroxisomes. Upon stimulation with agonists that induce Ca2+ release from intracellular stores, peroxisomes transiently take up Ca2+ reaching peak values in the lumen as high as 50-100 microm, depending on cell types. Also in resting cells, peroxisomes sustain a Ca2+ gradient, [Ca2+](perox) being approximately 20-fold higher than [Ca2+] in the cytosol ([Ca2+](cyt)). The properties of Ca2+ traffic across the peroxisomal membrane are different from those reported for other subcellular organelles. The sensitivity of peroxisomal Ca2+ uptake to agents dissipating H+ and Na+ gradients unravels the existence of a complex bioenergetic framework including V-ATPase, Ca2+/H+, and Ca2+/Na+ activities whose components are yet to be identified at a molecular level. The different [Ca2+](perox) of resting and stimulated cells suggest that Ca2+ could play an important role in the regulation of peroxisomal metabolism.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

