Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin
- PMID: 18364389
- PMCID: PMC2584350
- DOI: 10.1093/hmg/ddn092
Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin
Abstract
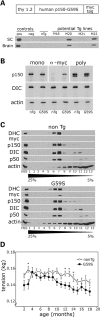
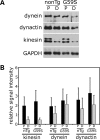
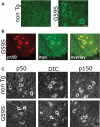
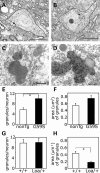
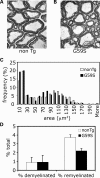
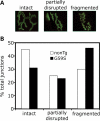
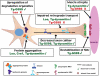
An increasing number of neurodegenerative diseases are being linked to mutations in genes encoding proteins required for axonal transport and intracellular trafficking. A mutation in p150(Glued), a component of the cytoplasmic dynein/dynactin microtubule motor complex, results in the human neurodegenerative disease distal spinal and bulbar muscular atrophy (dSBMA). We have developed a transgenic mouse model of dSBMA; these mice exhibit late-onset, slowly progressive muscle weakness but do not have a shortened lifespan, consistent with the human phenotype. Examination of motor neurons from the transgenic model reveals the proliferation of enlarged tertiary lysosomes and lipofuscin granules, indicating significant alterations in the cellular degradative pathway. In addition, we observe deficits in axonal caliber and neuromuscular junction (NMJ) integrity, indicating distal degeneration of motor neurons. However, sciatic nerve ligation studies reveal that inhibition of axonal transport is not evident in this model. Together, these data suggest that mutant p150(Glued) causes neurodegeneration in the absence of significant changes in axonal transport, and therefore other functions of dynein/dynactin, such as trafficking in the degradative pathway and stabilization of the NMJ are likely to be critical in maintaining the health of motor neurons.
Figures
References
-
- Chevalier-Larsen E., Holzbaur E.L. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta. 2006;1762:1094–1108. - PubMed
-
- Puls I., Jonnakuty C., LaMonte B.H., Holzbaur E.L., Tokito M., Mann E., Floeter M.K., Bidus K., Drayna D., Oh S.J., et al. Mutant dynactin in motor neuron disease. Nat. Genet. 2003;33:455–456. - PubMed
-
- Levy J.R., Sumner C.J., Caviston J.P., Tokito M.K., Ranganathan S., Ligon L.A., Wallace K.E., LaMonte B.H., Harmison G.G., Puls I., et al. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J. Cell Biol. 2006;172:733–745. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

