Neuroarchitecture of aminergic systems in the larval ventral ganglion of Drosophila melanogaster
- PMID: 18365004
- PMCID: PMC2268740
- DOI: 10.1371/journal.pone.0001848
Neuroarchitecture of aminergic systems in the larval ventral ganglion of Drosophila melanogaster
Abstract
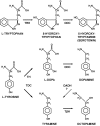
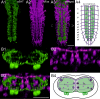
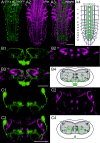
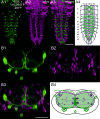
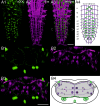
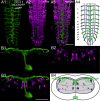
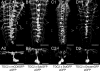
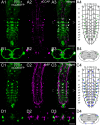
Biogenic amines are important signaling molecules in the central nervous system of both vertebrates and invertebrates. In the fruit fly Drosophila melanogaster, biogenic amines take part in the regulation of various vital physiological processes such as feeding, learning/memory, locomotion, sexual behavior, and sleep/arousal. Consequently, several morphological studies have analyzed the distribution of aminergic neurons in the CNS. Previous descriptions, however, did not determine the exact spatial location of aminergic neurite arborizations within the neuropil. The release sites and pre-/postsynaptic compartments of aminergic neurons also remained largely unidentified. We here used gal4-driven marker gene expression and immunocytochemistry to map presumed serotonergic (5-HT), dopaminergic, and tyraminergic/octopaminergic neurons in the thoracic and abdominal neuromeres of the Drosophila larval ventral ganglion relying on Fasciclin2-immunoreactive tracts as three-dimensional landmarks. With tyrosine hydroxylase- (TH) or tyrosine decarboxylase 2 (TDC2)-specific gal4-drivers, we also analyzed the distribution of ectopically expressed neuronal compartment markers in presumptive dopaminergic TH and tyraminergic/octopaminergic TDC2 neurons, respectively. Our results suggest that thoracic and abdominal 5-HT and TH neurons are exclusively interneurons whereas most TDC2 neurons are efferent. 5-HT and TH neurons are ideally positioned to integrate sensory information and to modulate neuronal transmission within the ventral ganglion, while most TDC2 neurons appear to act peripherally. In contrast to 5-HT neurons, TH and TDC2 neurons each comprise morphologically different neuron subsets with separated in- and output compartments in specific neuropil regions. The three-dimensional mapping of aminergic neurons now facilitates the identification of neuronal network contacts and co-localized signaling molecules, as exemplified for DOPA decarboxylase-synthesizing neurons that co-express crustacean cardioactive peptide and myoinhibiting peptides.
Conflict of interest statement
Figures


Similar articles
-
Neuroarchitecture of peptidergic systems in the larval ventral ganglion of Drosophila melanogaster.PLoS One. 2007 Aug 1;2(8):e695. doi: 10.1371/journal.pone.0000695. PLoS One. 2007. PMID: 17668072 Free PMC article.
-
Biogenic amine systems in the fruit fly Drosophila melanogaster.Microsc Res Tech. 1999 Apr 15;45(2):106-21. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. Microsc Res Tech. 1999. PMID: 10332728 Review.
-
Localization of the contacts between Kenyon cells and aminergic neurons in the Drosophila melanogaster brain using SplitGFP reconstitution.J Comp Neurol. 2013 Dec 1;521(17):3992-4026. doi: 10.1002/cne.23388. J Comp Neurol. 2013. PMID: 23784863
-
Tyrosine hydroxylase- and serotonin-immunoreactive neurons in the thoracic ganglia of the Oriental fruit fly, Bactrocera dorsalis.Arthropod Struct Dev. 2025 May;86:101444. doi: 10.1016/j.asd.2025.101444. Epub 2025 Apr 1. Arthropod Struct Dev. 2025. PMID: 40174558
-
Cytoarchitecture of histamine-, dopamine-, serotonin- and octopamine-containing neurons in the cricket ventral nerve cord.Microsc Res Tech. 1999 Jan 15-Feb 1;44(2-3):137-65. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<137::AID-JEMT7>3.0.CO;2-3. Microsc Res Tech. 1999. PMID: 10084822 Review.
Cited by
-
A LAT1-Like Amino Acid Transporter Regulates Neuronal Activity in the Drosophila Mushroom Bodies.Cells. 2024 Aug 13;13(16):1340. doi: 10.3390/cells13161340. Cells. 2024. PMID: 39195231 Free PMC article.
-
Systematic analysis of fly models with multiple drivers reveals different effects of ataxin-1 and huntingtin in neuron subtype-specific expression.PLoS One. 2014 Dec 31;9(12):e116567. doi: 10.1371/journal.pone.0116567. eCollection 2014. PLoS One. 2014. PMID: 25551764 Free PMC article.
-
The role of dopamine in Drosophila larval classical olfactory conditioning.PLoS One. 2009 Jun 12;4(6):e5897. doi: 10.1371/journal.pone.0005897. PLoS One. 2009. PMID: 19521527 Free PMC article.
-
Visualisation of ribosomes in Drosophila axons using Ribo-BiFC.Biol Open. 2020 Jan 2;8(12):bio047233. doi: 10.1242/bio.047233. Biol Open. 2020. PMID: 31822474 Free PMC article.
-
Cell type-specific genomics of Drosophila neurons.Nucleic Acids Res. 2012 Oct;40(19):9691-704. doi: 10.1093/nar/gks671. Epub 2012 Aug 1. Nucleic Acids Res. 2012. PMID: 22855560 Free PMC article.
References
-
- Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–121. - PubMed
-
- Nässel DR. Neuropeptides, amines and amino acids in an elementary insect ganglion: functional and chemical anatomy of the unfused abdominal ganglion. Prog Neurobiol. 1996;48:325–420. - PubMed
-
- Pflüger HJ, Stevenson PA. Evolutionary aspects of octopaminergic systems with emphasis on arthropods. Arthropod Structure & Development. 2005;34:379–396.
-
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

