Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7
- PMID: 18365021
- PMCID: PMC2267223
- DOI: 10.1371/journal.pone.0001876
Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7
Abstract
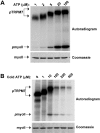
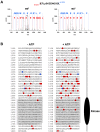
TRPM6 and TRPM7 are bifunctional proteins expressing a TRP channel fused to an atypical alpha-kinase domain. While the gating properties of TRPM6 and TRPM7 channels have been studied in detail, little is known about the mechanisms regulating kinase activity. Recently, we found that TRPM7 associates with its substrate myosin II via a kinase-dependent mechanism suggesting a role for autophosphorylation in substrate recognition. Here, we demonstrate that the cytosolic C-terminus of TRPM7 undergoes massive autophosphorylation (32+/-4 mol/mol), which strongly increases the rate of substrate phosphorylation. Phosphomapping by mass spectrometry indicates that the majority of autophosphorylation sites (37 out of 46) map to a Ser/Thr-rich region immediately N-terminal of the catalytic domain. Deletion of this region prevents substrate phosphorylation without affecting intrinsic catalytic activity suggesting that the Ser/Thr-rich domain contributes to substrate recognition. Surprisingly, the TRPM6-kinase is regulated by an analogous mechanism despite a lack of sequence conservation with the TRPM7 Ser/Thr-rich domain. In conclusion, our findings support a model where massive autophosphorylation outside the catalytic domain of TRPM6 and TRPM7 may facilitate kinase-substrate interactions leading to enhanced phosphorylation of those substrates.
Conflict of interest statement
Figures



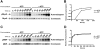


References
-
- Bodding M. TRPM6: A Janus-like protein. Handb Exp Pharmacol. 2007;179:299–311. - PubMed
-
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. - PubMed
-
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

