The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease
- PMID: 18365243
- PMCID: PMC2531252
- DOI: 10.1007/s00424-008-0491-8
The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease
Abstract
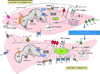
Calcium (Ca(2+)) is a highly versatile second messenger that controls vascular smooth muscle cell (VSMC) contraction, proliferation, and migration. By means of Ca(2+) permeable channels, Ca(2+) pumps and channels conducting other ions such as potassium and chloride, VSMC keep intracellular Ca(2+) levels under tight control. In healthy quiescent contractile VSMC, two important components of the Ca(2+) signaling pathways that regulate VSMC contraction are the plasma membrane voltage-operated Ca(2+) channel of the high voltage-activated type (L-type) and the sarcoplasmic reticulum Ca(2+) release channel, Ryanodine Receptor (RyR). Injury to the vessel wall is accompanied by VSMC phenotype switch from a contractile quiescent to a proliferative motile phenotype (synthetic phenotype) and by alteration of many components of VSMC Ca(2+) signaling pathways. Specifically, this switch that culminates in a VSMC phenotype reminiscent of a non-excitable cell is characterized by loss of L-type channels expression and increased expression of the low voltage-activated (T-type) Ca(2+) channels and the canonical transient receptor potential (TRPC) channels. The expression levels of intracellular Ca(2+) release channels, pumps and Ca(2+)-activated proteins are also altered: the proliferative VSMC lose the RyR3 and the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase isoform 2a pump and reciprocally regulate isoforms of the ca(2+)/calmodulin-dependent protein kinase II. This review focuses on the changes in expression of Ca(2+) signaling proteins associated with VSMC proliferation both in vitro and in vivo. The physiological implications of the altered expression of these Ca(2+) signaling molecules, their contribution to VSMC dysfunction during vascular disease and their potential as targets for drug therapy will be discussed.
Conflict of interest statement
Figures
References
-
- Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–584. - PubMed
-
- Abraham ST, Shaw C. Increased expression of deltaCaMKII isoforms in skeletal muscle regeneration: Implications in dystrophic muscle disease. J Cell Biochem. 2006;97:621–632. - PubMed
-
- Abramowitz J, Aydemir-Koksoy A, Helgason T, Jemelka S, Odebunmi T, Seidel CL, Allen JC. Expression of plasma membrane calcium ATPases in phenotypically distinct canine vascular smooth muscle cells. J Mol Cell Cardiol. 2000;32:777–789. - PubMed
-
- Afroze T, Husain M. Cell cycle dependent regulation of intracellular calcium concentration in vascular smooth muscle cells: a potential target for drug therapy. Current Drug Targets. 2001;1:23–40. - PubMed
-
- Afroze T, Sadi AM, Momen MA, Gu S, Heximer S, Husain M. c-Myb-dependent inositol 1,4,5-trisphosphate receptor type-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1305–1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

