A novel function of dcf1 during the differentiation of neural stem cells in vitro
- PMID: 18365309
- PMCID: PMC11515049
- DOI: 10.1007/s10571-008-9266-1
A novel function of dcf1 during the differentiation of neural stem cells in vitro
Abstract
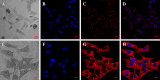
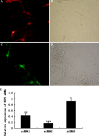
The study of neural dendrite formation is of great significance both in theory and applications. However, the molecular mechanisms of regulation remain unclear. We previously described a novel EST, which has high homology with dentritic cell factors (DCF1), expressed differentially between undifferentiated and differentiated neural stem cells (NSCs). In this study, we cloned, expressed, and silenced the dcf1 gene and offered insight into its function in regulating dendrite formation during the differentiation of NSCs. The results indicated that dcf1 encoded a 42 kD protein and could be successfully expressed both in Escherichia coli and NSCs. In order to silence dcf1 gene, three different kinds of siRNA vectors were constructed and transformed into the NSC line C17.2 and primary NSCs, resulting in down regulation of the dcf1 mRNA. Analysis of immunofluorescence or GFP illuminated that with overexpression of the dcf1 gene, the NSCs were maintained in undifferentiated status. After the dcf1 gene was silenced, cells tended to differentiate into neurons and astrocytes.
Figures



References
-
- Arenas E et al (2002) Stem cells in the treatment of Parkinson’s disease. Brain Res Bull 57:795–808 - PubMed
-
- Arne C et al (2003) wnt1 and wnt10b function redundantly at the zebrafish midbrain–hindbrain boundary. Dev Biol 254:172–187 - PubMed
-
- Dietz AB, Bulur PA, Knutson GJ, Matasic R, Vuk-Pavlovic S et al (2000) Maturation of human monocyte-derived dendritic cells studied by microarray hybridization. Biochem Biophys Res Commun 275:731–738 - PubMed
-
- Edlund T et al (1999) Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96:211–224 - PubMed
-
- Engleka KA et al (2005) Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol 280:396–406 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

