Medical biofilms
- PMID: 18366134
- PMCID: PMC2706312
- DOI: 10.1002/bit.21838
Medical biofilms
Abstract
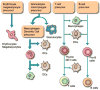
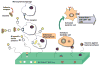
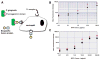
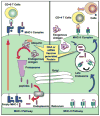
For more than two decades, Biotechnology and Bioengineering has documented research focused on natural and engineered microbial biofilms within aquatic and subterranean ecosystems, wastewater and waste-gas treatment systems, marine vessels and structures, and industrial bioprocesses. Compared to suspended culture systems, intentionally engineered biofilms are heterogeneous reaction systems that can increase reactor productivity, system stability, and provide inherent cell:product separation. Unwanted biofilms can create enormous increases in fluid frictional resistances, unacceptable reductions in heat transfer efficiency, product contamination, enhanced material deterioration, and accelerated corrosion. Missing from B&B has been an equivalent research dialogue regarding the basic molecular microbiology, immunology, and biotechnological aspects of medical biofilms. Presented here are the current problems related to medical biofilms; current concepts of biofilm formation, persistence, and interactions with the host immune system; and emerging technologies for controlling medical biofilms.
Copyright 2007 Wiley Periodicals, Inc.
Figures





References
-
- An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43(3):338–348. - PubMed
-
- Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110.
-
- Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: The type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. - PubMed
-
- Atkinson RA, Salah El Din AL, Kieffer B, Lefevre JF, Abdallah MA. Bacterial iron transport: 1H NMR determination of the three-dimensional structure of the gallium complex of pyoverdin G4R, the peptidic siderophore of Pseudomonas putida G4R. Biochemistry. 1998;37:15965–15973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

