The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses
- PMID: 18366184
- PMCID: PMC2902722
- DOI: 10.1021/bi7023354
The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses
Abstract
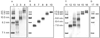
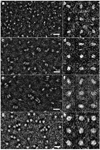
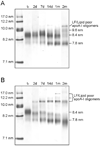
High-density lipoprotein (HDL) mediates reverse cholesterol transport (RCT), wherein excess cholesterol is conveyed from peripheral tissues to the liver and steroidogenic organs. During this process HDL continually transitions between subclass sizes, each with unique biological activities. For instance, RCT is initiated by the interaction of lipid-free/lipid-poor apolipoprotein A-I (apoA-I) with ABCA1, a membrane-associated lipid transporter, to form nascent HDL. Because nearly all circulating apoA-I is lipid-bound, the source of lipid-free/lipid-poor apoA-I is unclear. Lecithin:cholesterol acyltransferase (LCAT) then drives the conversion of nascent HDL to spherical HDL by catalyzing cholesterol esterification, an essential step in RCT. To investigate the relationship between HDL particle size and events critical to RCT such as LCAT activation and lipid-free apoA-I production for ABCA1 interaction, we reconstituted five subclasses of HDL particles (rHDL of 7.8, 8.4, 9.6, 12.2, and 17.0 nm in diameter, respectively) using various molar ratios of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, free cholesterol, and apoA-I. Kinetic analyses of this comprehensive array of rHDL particles suggest that apoA-I stoichiometry in rHDL is a critical factor governing LCAT activation. Electron microscopy revealed specific morphological differences in the HDL subclasses that may affect functionality. Furthermore, stability measurements demonstrated that the previously uncharacterized 8.4 nm rHDL particles rapidly convert to 7.8 nm particles, concomitant with the dissociation of lipid-free/lipid-poor apoA-I. Thus, lipid-free/lipid-poor apoA-I generated by the remodeling of HDL may be an essential intermediate in RCT and HDL's in vivo maturation.
Figures



References
-
- Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36:211–228. - PubMed
-
- Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 2005;85:1343–1372. - PubMed
-
- von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler., Thromb., Vasc. Biol. 2001;21:13–27. - PubMed
-
- Jonas A. Regulation of lecithin cholesterol acyltransferase activity. Prog. Lipid Res. 1998;37:209–234. - PubMed
-
- Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999;22:347–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

