Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice
- PMID: 18366296
- PMCID: PMC2649688
- DOI: 10.1089/scd.2008.0018
Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice
Abstract
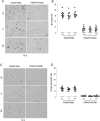
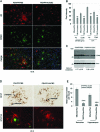
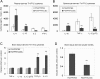
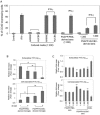
Modulation of immune/inflammatory responses by diverse strategies including amyloid-beta (Abeta) immunization, nonsteroidal anti-inflammatory drugs, and manipulation of microglial activation states has been shown to reduce Alzheimer's disease (AD)-like pathology and cognitive deficits in AD transgenic mouse models. Human umbilical cord blood cells (HUCBCs) have unique immunomodulatory potential. We wished to test whether these cells might alter AD-like pathology after infusion into the PSAPP mouse model of AD. Here, we report a marked reduction in Abeta levels/beta-amyloid plaques and associated astrocytosis following multiple low-dose infusions of HUCBCs. HUCBC infusions also reduced cerebral vascular Abeta deposits in the Tg2576 AD mouse model. Interestingly, these effects were associated with suppression of the CD40-CD40L interaction, as evidenced by decreased circulating and brain soluble CD40L (sCD40L), elevated systemic immunoglobulin M (IgM) levels, attenuated CD40L-induced inflammatory responses, and reduced surface expression of CD40 on microglia. Importantly, deficiency in CD40 abolishes the effect of HUCBCs on elevated plasma Abeta levels. Moreover, microglia isolated from HUCBC-infused PSAPP mice demonstrated increased phagocytosis of Abeta. Furthermore, sera from HUCBC-infused PSAPP mice significantly increased microglial phagocytosis of the Abeta1-42 peptide while inhibiting interferon-gammainduced microglial CD40 expression. Increased microglial phagocytic activity in this scenario was inhibited by addition of recombinant CD40L protein. These data suggest that HUCBC infusion mitigates AD-like pathology by disrupting CD40L activity.
Figures



References
-
- Benzing WC. Wujek JR. Ward EK. Shaffer D. Ashe KH. Younkin SG. Brunden KR. Evidence for glialmediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. - PubMed
-
- Bard F. Cannon C. Barbour R. Burke RL. Games D. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Lieberburg I. Motter R. Nguyen M. Soriano F. Vasquez N. Weiss K. Welch B. Seubert P. Schenk D. Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 2000;6:916–919. - PubMed
-
- Nicoll JA. Wilkinson D. Holmes C. Steart P. Markham H. Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nature Med. 2003;9:448–452. - PubMed
-
- Schenk D. Barbour R. Dunn W. Gordon G. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Liao Z. Lieberburg I. Motter R. Mutter L. Soriano F. Shopp G. Vasquez N. Vandevert C. Walker S. Wogulis M. Yednock T. Games D. Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

