Isolation of human foetal myoblasts and its application for microencapsulation
- PMID: 18366454
- PMCID: PMC3823488
- DOI: 10.1111/j.1582-4934.2007.00119.x
Isolation of human foetal myoblasts and its application for microencapsulation
Abstract
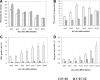
Foetal cells secrete more growth factors, generate less immune response, grow and proliferate better than adult cells. These characteristics make them desirable for recombinant modification and use in microencapsulated cellular gene therapeutics. We have established a system in vitro to obtain a pure population of primary human foetal myoblasts under several rounds of selection with non-collagen coated plates and identified by desmin staining. These primary myoblasts presented good proliferation ability and better differentiation characteristics in monolayer and after microencapsulation compared to murine myoblast C2C12 cells based on creatine phosphokinase (CPK), major histocompatibility complex (MHC) and multi-nucleated myotubule determination. The lifespan of primary myoblasts was 70 population doublings before entering into senescent state, with a population time of 18-24 hrs. Hence, we have developed a protocol for isolating human foetal primary myoblasts with excellent differentiation potential and robust growth and longevity. They should be useful for cell-based therapy in human clinical applications with microencapsulation technology.
Figures




References
-
- Emmrich F, Keitel R, Sorger K, Otto U, Staffa G, Klotzer B. Quantitative alterations of immune serum globulin concentrations in pigs transplanted with a renal allograft. Exp Pathol. 1977;14:334–9. - PubMed
-
- Statter MB, Fahrner KJ, Barksdale EM, Jr, Parks DE, Flavell RA, Donahoe PK. Correlation of fetal kidney and testis congenic graft survival with reduced major histocompatibility complex burden. Transplantation. 1989;47:651–60. - PubMed
-
- Nouwen EJ, Dauwe S, Van Der Biest I, De Broe ME. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int. 1993;44:147–58. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

